This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Sara Christine Norkelun/Sandbox 1
From Proteopedia
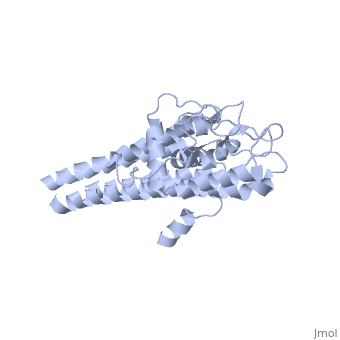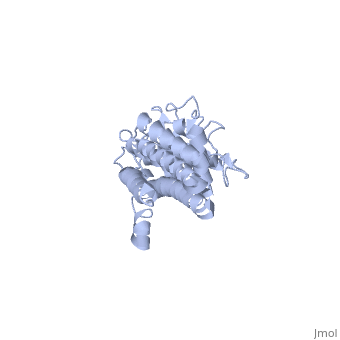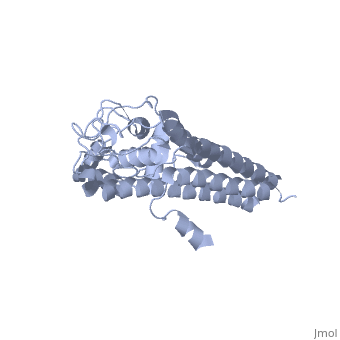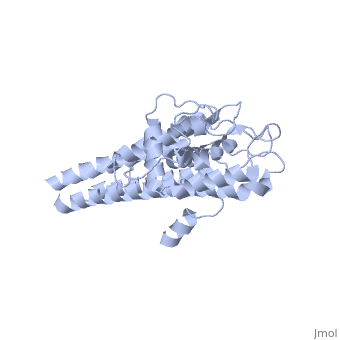
VlsE
Vmp-like sequence, Expressed
|
Contents |
Introduction
Lyme disease is a systemic bacterial infection that can lead to chronic debilitating effects if left untreated. The symptoms are varied and affect multiple systems, sometimes making diagnosis difficult. Some initial symptoms are not unique to Lyme disease, and include fever, headache, lethargy, and soreness. The main identifying symptom is a characteristic bullseye rash that develops locally near the site of infection. If the infection is allowed to progress, more serious symptoms develop such as arthritis, swollen joints, and even neurological symptoms such as depression and psychosis.
In most of the Eastern region of North America, the disease is primarily caused by the spirochete bacterium, Borellia burgdorferi. B. burgdorferi has a complex life cycle, transferring among different hosts. However, the main vector of disease transmission to humans is the deer tick, Ixodes scapularis, particularly the young nymph stage.
The main challenge to treatment of the disease is the bacteria's lengthy incubation time. After the time of entry, weeks may pass before symptoms begin to emerge, many of which are indistinguishable from more common maladies such as a cold or fatigue. During these initial weeks, Borellia burgdorferi is able to evade immune response for reasons which are not completely understood. It appears that this allows the bacteria to spread systemically and effect sites distal to the infection site, such as the joints and the central nervous system.
Immune Evasion
The evasive nature of the pathogen Borrelia burgdorferi is believed to be due in part to its unique surface antigen, , or "Vmp-like sequence, Expressed". VlsE belongs to a class of proteins known as variable surface antigens, meaning that they are antigens located on the outer surface of the membrane, but are also of a variable nature. VlsE is a lipoprotein that is abundant on the bacterial membrane. The protein has extremely variable regions of high immunogenicity, along with invariable regions that, while immuno-reactive in-vitro, are shielded from antibody in-vivo[1]. This unique blend of sequence variability and structural shielding is what is responsible for the proliferative nature of the bacteria in the human body, through its ability to evade immune recognition.
VlsE may serve other unknown functions, but there is support for its role in immune evasion. If B. burgdorferi loses the VlsE-encoding plasmid Ip28-1, the result is a significantly reduced degree of infection in the mouse model[2]. When B. burgdorferi enters a mammalian host, VlsE expression increases, as does the generation of sequence variability in VlsE due to recombination[1].
This property is a result of the antigen's complex structure, which is comprised of a proximal region which is presumably embedded in the bacteria's membrane, and an exposed distal region. The distal exposed region is the area of most interest, because it is comprised of small alternating variable and invariable regions. While the invariable regions maintain a constant amino acid sequence between generations of bacteria, the variable regions undergo a complex gene recombination mechanism which alters the variable regions between generations[1]. These rapid modifications in the variable regions may confer the bacteria with a kind of protection from immune recognition. The constantly changing variable regions on the exposed surface may act as a mask, preventing antibodies from making a reliable identification.
Antibody Recognition
Antibody recognition of VlsE is difficult, because genetic recombination is constantly altering the residues in the exposed variable regions, making the antigen appear different and unidentifiable. Therefore, if an antibody does match a variable region on VlsE and binds, its effectiveness will become null within several generations of cell division and recombination. There is, however, one invariable region, IR6, that has been shown to elicit a strong antibody response in vitro, and is the basis of a serological diagnostic test for Lyme. IR6, is one of the 6 invariable regions contained within the variable domain of the antigen. Serum samples from rhesus monkeys analyzed through a peptide based ELISA assay showed a strong antibody response to IR6. The antigenticity of this particular region was confirmed because none of the other IR regions (IR1-IR5) elicited a strong antibody response [3].
There is also an indication that the formation of in vivo may be responsible for some sheilding of the invariable regions at the connection between the two monomers[1]. This may help to further explain why IR6, although immunogenic when isolated, seems inaccessable to antibodies when integrated on the bacterial membrane.
Mechanism of genetic recombination within variable regions
VlsE is composed of a variable domain (residues 118-310) flanked at the N and C termini by invariable domains. On the plasmid, the VlsE gene is followed by 15 unexpressed copies the variable domain. These unexpressed copies vary in sequence, and during cell division parts of these can recombine into the expressed VlsE gene. This leads to rapid amino acid sequence divergence within the mammalian host, far faster than mutation. Changes are detectable as soon as 4 days post-infection, and can comprise 9-13 recombinations within a single host within a month[1]. The result is the generation of vast antigenic diversity, estimated to be orders of magnitude larger than the diversity of vertebrate antibodies[4].
Protein structure
When VlsE is folded into its tertiary structure, the consists of four alpha-helical regions proximal to the spirochete membrane thought to be relatively inaccessible to host antibody. The invariable domain is membrane-distal and more exposed to antibodies[1].
Within the membrane-distal are six alternating invariable regions (IR) and variable regions (VR), each consisting of sequences of four to thirty amino acids. The IR sequences contain alpha helical structures, while the VR sequences form loops with no secondary structure. The loops of the VR regions are more surface-exposed than the IR regions, and this is thought to physically the IR regions from antibody[1]. Of the 25 residues of the immunodominant IR6 region, Eicken et. al. (2002) suggest that only (lys 276, gln 279, lys 291, and lys 294) are involved in epitope binding[1].
Clinical Relevance
ELISA test
The only diagnostically sufficient clinical sign for Lyme disease is the presence of Erythema Migrans rash. The rash does not happen in all cases, however, and when present, it is only in the early stages of infection. Other symptoms of Lyme, such as fever, neurological signs, and joint inflammatio, are non-specific to the disease. Because of this, antigenic testing is an important component of Lyme diagnosis[5]. The first tier test for Lyme is often the C6 ELISA, which uses as its antigen C6, a manufactured peptide homologous to IR6[6]. IR6 has several qualities that make it a good antigen for a single-epitope immunologic test such as ELISA: (1) most patients with Lyme produce detectable levels of antibody to IR6, (2) the antigenic epitope is conserved so that one antigenic test sequence suffices regardless of the strain of B. burgdorferi being tested for, and (3) the antibody is not generally found in patients without a history of Lyme. These characteristics allow C6 to be a sensitive test for Lyme disease, with 74% of acute phase infections and over 90% of convalescent and late-phase infections yielding positive results[6]. C6 also results in good test specificity, with a 1.1% rate of false positives among patients with relapsing fever and autoimmune conditions (symptoms which can be mistaken for Lyme), patients with syphilis (caused by the sister genus to Borrelia), and patients drawn at random from areas to which Lyme is not endemic[6].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Eicken, C., Sharma, V., Klabunde, T., Lawrenz, M. B., Hardham, J. M., Norris, S. J., and Sacchettini, J. C. (2002) J. Bio. Chemistry 227:24 21691-21696.
- ↑ Labandiera-Rey, M., Baker, E., and Skare, J. T. (2001) J. Infect. Immun. 446-455.
- ↑ Liang, F. T., Phillip, M. T. (1999) Infection and Immunity" 67:12 6702-6706.
- ↑ Liang FT, Alvarez AL, Gu Y, Nowling JM, Ramamoorthy R, Philipp MT. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol. 1999 Nov 15;163(10):5566-73. PMID:10553085
- ↑ Gomes-Solecki MJ, Meirelles L, Glass J, Dattwyler RJ. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin Vaccine Immunol. 2007 Jul;14(7):875-9. Epub 2007 May 30. PMID:17538122 doi:10.1128/CVI.00122-07
- ↑ 6.0 6.1 6.2 Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol 37(12):3990-6.