User:Taranvir Rattu/Sandbox 1
From Proteopedia
Contents |
LHPP
|
Function
Phospholysine Phosphohistidine Inorganic Pyrophosphate Phosphatase (LHPP) is a 59.14 kDa homodimer phosphatase with an evolutionary presence that traces back to bacteria into modern humans. LHPP’s primary function is to catalyze the removal of phosphates from the nitrogen containing amino acids Lysine and Histidine, as well as the division of pyrophosphate into individual phosphates. As such, it belongs to the HAD-like hydrolase family. LHPP does this with the additional help from Mg2+ ions found in each subunit. For humans, expression is high in the brain, kidney, and liver tissues. Within the cell itself, LHPP is found to be a cytosolic and nuclear protein. Its metabolic involvement in the human body has been elucidated to be part of nucleobase synthesis and tumor suppression, however its concerted involvement is unknown.
Mechanism
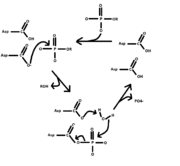
- HAD-like hydrolases have a unique characteristic about their mechanism, setting them apart from alkaline or tyrosine-specific hydrolases. Instead of utilizing a serine or cysteine as the nucleophile, HAD-like hydrolases use an aspartate residue. Additionally, another aspartate is present to aid in stabilization of the mechanism. As seen in figure 1, the aspartate residue performs nucleophilic attack on the phosphate, releasing the adjacent R-group. The R group is also protonated by the adjacent aspartic acid residue. The adjacent aspartic acid residue then functions as a base on an incoming H2O molecule, converting it into a hydroxide nucleophile and regenerating the aspartic acid. The hydroxide performs nucleophilic attack on the phosphate, liberating and renewing the enzyme.
Structural Description
- LHPP is a homodimer with cyclic C2 symmetry. There are 4 total domains within the enzyme, all primarily consisting of Rossmainoid folds forming a 3 stacked 𝜶/𝜷 sandwich, with a pattern of repeating 𝜷-𝜶 units. The core catalytic domain contains 5 B-strands with a spacial alignment of ‘54123’. With HAD-like hydrolases, the Rossman fold helps form distinct conformations states that contribute to its substrate specificity and active site availability, which are known as elements.
Squiggle and Flap
- The initial mechanism requires first solvent exclusion to form a favorable nucleophilic attack by the Asp residue, followed by solvent inclusion for the subsequent hydrolysis. Thus, the swapping between these states is crucial for catalytic activity. Highlighted in the figure, the squiggle elements, consisting of 6 amino acids, form a helical turn and the flap element, located downstream of the squiggle, adopts a B-hairpin turn. The helical squiggle alternates between tight or loose conformations, which then triggers the movement of the flap. The B-hairpin, due to its positioning, can partially cover the active site.
Cap Domain
- are a conserved structural element in HAD-like hydrolases. As such, LHPP has a cap domain as well, subtype C2. As seen adjacent, the cap domain for LHPP is a 𝜷-sandwich with 3 𝜷 strands and 𝜶-helices. Without the C2 cap domain, the catalytic site would be open and it would function on macromolecular substrates, however the steric hindrance of the domain by the C2 cap restricts it to smaller molecules.
Motifs
- The catalytic domain of HAD-like hydrolases are highly conserved in the family, with 4 important motifs present. The first HAD signature motif is ‘hhhDxDx’. The two aspartic acid residues function to coordinate the Mg2+ ion present in the core. HAD signature motif 2 is ‘hhhhhh(S/T)’ with a serine or threonine serving to form a hydrogen bond so that the substrate can orient itself in the correct direction. HAD signature motif 3 is simply a lysine residue spaced out 18-30 residues from motif 4. This motif functions to coordinate with the serine/threonine residue to stabilize the negative charge on the reaction intermediate. The final HAD motif is (G/S)(D/S)xxx(D/E)hhhh. Again, these residues are responsible for additional coordination of the Mg2+ ion.
Pathology
- LHPP has not been well characterized in human pathology. It has been identified as a tumor suppressor in hepatic tissue in mice and low expression was correlated with hepatocellular carcinoma. Oddly enough, it has also been associated with mental health as well, such as Major Depressive Disorder, risky sexual behaviors, and alcohol dependence. Further research is still required on these subjects.
References
References Bioinformatics, C. (2020). Retrieved 30 April 2020, from https://www.nextprot.org/entry/NX_Q9H008/structures
Burroughs, A., Allen, K., Dunaway-Mariano, D., & Aravind, L. (2006). Evolutionary Genomics of the HAD Superfamily: Understanding the Structural Adaptations and Catalytic Diversity in a Superfamily of Phosphoesterases and Allied Enzymes. Journal Of Molecular Biology, 361(5), 1003-1034. doi: 10.1016/j.jmb.2006.06.049
Gohla, A. (2019). Do metabolic HAD phosphatases moonlight as protein phosphatases?. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research, 1866(1), 153-166. doi: 10.1016/j.bbamcr.2018.07.007
Hindupur, S., Colombi, M., Fuhs, S., Matter, M., Guri, Y., & Adam, K. et al. (2018). The protein histidine phosphatase LHPP is a tumour suppressor. Nature, 555(7698), 678-682. doi: 10.1038/nature26140
Hiraishi, H., Yokoi, F., & Kumon, A. (1998). 3-Phosphohistidine and 6-Phospholysine Are Substrates of a 56-kDa Inorganic Pyrophosphatase from Bovine Liver. Archives Of Biochemistry And Biophysics, 349(2), 381-387. doi: 10.1006/abbi.1997.0480
Koike, E., Toda, S., Yokoi, F., Izuhara, K., Koike, N., & Itoh, K. et al. (2006). Expression of new human inorganic pyrophosphatase in thyroid diseases: Its intimate association with hyperthyroidism. Biochemical And Biophysical Research Communications, 341(3), 691-696. doi: 10.1016/j.bbrc.2006.01.016
LHPP phospholysine phosphohistidine inorganic pyrophosphate phosphatase [Homo sapiens (human)] - Gene - NCBI. (2020). Retrieved 30 April 2020, from https://www.ncbi.nlm.nih.gov/gene/64077
PDB 2x4d. (2020). Retrieved 30 April 2020, from http://cathdb.info/pdb/2x4d
Seifried, A., Schultz, J., & Gohla, A. (2012). Human HAD phosphatases: structure, mechanism, and roles in health and disease. FEBS Journal, 280(2), 549-571. doi: 10.1111/j.1742-4658.2012.08633.x
Yokoi, F. (2003). Molecular Cloning of a cDNA for the Human Phospholysine Phosphohistidine Inorganic Pyrophosphate Phosphatase. Journal Of Biochemistry, 133(5), 607-614. doi: 10.1093/jb/mvg078
Cui, L., Gong, X., Tang, Y., Kong, L., Chang, M., & Geng, H. et al. (2016). Relationship between the LHPP Gene Polymorphism and Resting-State Brain Activity in Major Depressive Disorder. Neural Plasticity, 2016, 1-8. doi: 10.1155/2016/9162590
Koike, E., Toda, S., Yokoi, F., Izuhara, K., Koike, N., & Itoh, K. et al. (2006). Expression of new human inorganic pyrophosphatase in thyroid diseases: Its intimate association with hyperthyroidism. Biochemical And Biophysical Research Communications, 341(3), 691-696. doi: 10.1016/j.bbrc.2006.01.016
Neff, C., Abkevich, V., Packer, J., Chen, Y., Potter, J., & Riley, R. et al. (2008). Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Molecular Psychiatry, 14(6), 621-630. doi: 10.1038/mp.2008.8
Polimanti, R., Wang, Q., Meda, S., Patel, K., Pearlson, G., & Zhao, H. et al. (2016). The Interplay Between Risky Sexual Behaviors and Alcohol Dependence: Genome-Wide Association and Neuroimaging Support for LHPP as a Risk Gene. Neuropsychopharmacology, 42(3), 598-605. doi: 10.1038/npp.2016.153