User:Yash Patankar/Sandbox 1
From Proteopedia
| |||||||||
| 2cg9, resolution 3.10Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Related: | 1a4h, 1ah6, 1ah8, 1am1, 1amw, 1bgq, 1hk7, 1us7, 1usu, 1usv, 2akp, 2brc, 2bre, 2cge | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Hsp90
Introduction
Hsp90, a 90 kDa heat-shock protein, is a ubiquitous molecular chaperone responsible for the governing of protein folding and regulation of many eukaryotic signaling pathways in the cell (Ali et al., 2006). It is a non-fibrous protein involved in the activation of many regulatory and signaling client proteins under normal conditions in addition to the refolding of a number of proteins during stress (Ali et al., 2006; Pearl et al., 2008). Hsp90 is one of the most abundant proteins expressed in cells and it has been estimated to constitute upto 2% of the cellular proteins (Dollins et al., 2007). Hsp90 is found in all eukaryotes as well as bacteria with the exception of archae (Chen et al., 2006). Hsp90 has been shown to act as a buffer or capacitor of genetic variation in morphological evolution in Drosophila and other model organisms (Rutherford et al., 2007). Hsp90 is known to be required for the function of protein kinases such as ErbB2m, Cdk4, B-Raf and Akt/protein kinase which are upregualted in cancerous cells (Pearl, 2005).
The function of Hsp90 depends on its ability to hydrolyze ATP after it binds in the active site of the protein and hence, the inhibition of Hsp90 by ATP competitors or non-hydrolyzable ATP analogs leads to the promotion of client degradation.
The client proteins for Hsp 90 can be classified as follows:
- Protein kinases – most coherent (ex. c-Src, PKB)for druge targeting
- Transcription factors (ex. nuclear receptors for steroid hormones)
- Viral-replication proteins, proteins involved in innate immunity
Structure
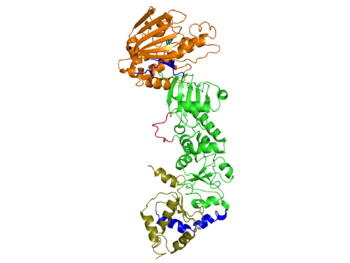
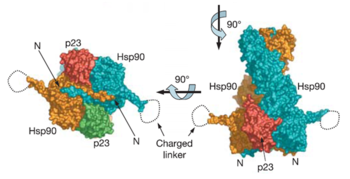
The Pearl group (2006) first determined the complete structure of yeast Hsp90 along with the co-chaperone p23 (mammals)/Sba1 (yeast homolog) bound to a non-hydrolyzable ATP analog. This structure was obtained using an Hsp90 mutant (A107N) that was previously shown by the Pearl group to not alter the function of the wild type protein. The PDB id for the protein is 2cg9 and the crystallographic information can be found on http://oca.weizmann.ac.il/oca-bin/ocaids.
Each Hsp90 monomer consists of an N-teminal domain, a middle (M) domain and a C-terminal domain. The N-terminal domain (a.a. 1-216) is formed by a twisted six stranded β-sheet on one face of the monomer and six α-helices, leading to a two-layer sandwich. The charged linker connecting the N-terminal and M domains consists of poorly conserved and low complexity repeats of amino acids and thus is an obstacle to obtaining crystals showing strong diffraction patterns and was thus omitted from the structure determination process and replaced by an eight residue-loop. The middle domain is subdivided into a large (a.a. 273-409) and small (a.a. 435-525) domain, both of which are α-β-α domains. The C-terminal domain (a.a. 525-709) consists of a three stranded β-sheet a five α-helices. A three-helix coil forms the constitutive dimerization interface for the protomer as seen in the figure for Hsp90 above. The residues 678-709 provide the binding sequence for TPR-domain co-chaperones, but they are not crystallized as they are highly disordered (Ali et al., 2006). Sba1/p23 molecules bind to Hsp90 in the junction of the N-terminal domains of the dimer. Figure shows bound Sba1/p23 and Hsp90.
Conformation of Hsp90
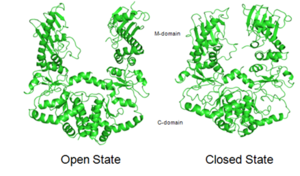
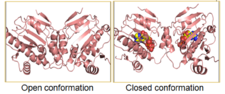
Hsp90 is observed in a distinct “closed” and an “open” conformation. Binding of ATP leads to the transient dimerization (homodimer) of Hsp90 with form the C-terminus domain. The C-terminal domains form a molecular clamp that leads to the dimerization. It is not clear whether the “open” conformation of the dimer exists in vivo as X-ray crystallization studies show that there is defined conformation for Hsp90 in vivo. However, the crystal structures for Hsp90 homologs which lack ATP show distinct conformations, as seen for HtpG, the bacterial Hsp90 homolog (Pearl et al., 2008).
- Molecular clamp
The interface between the C-terminal domain and the middle domain flexes upon the binding of ATP, leading to a huge twisted V conformational change that leads to the closure of the domains. The figures show the open as well as the closed state of Hsp90, only for the M and C domains. The structure for all the N, M and C domains in the open conformation is not available for Hsp90, but is available for its homologs such as bacterial HtpG (Pearl et al., 2008).
- N-terminal
The following changes take place in the N-terminal domain upon the binding of ATP:
- The lid segment (a.a. 94-125) swings by ~180o leading to the exposition of a hydrophobic patch on Leu 15 and Leu 18. In addition, the involvement of Gln 14, Thr 95, Ile 96, Ala 97 and Phe 120 leads to an interface between the equivalents on the other monomer (Ali et al., 2006).
- Thr 22, Val 23 and Tyr 24 form hydrophobic interactions with Leu 376 and Leu 378 from the middle domain (Ali et a., 2006).
- Phe 200 makes hydrophobic interactions with Thr 273, Pro 275, Trp 277, Phe 292 and Tyr 344 from the middle domain.
- Amino acid residues from 114 to 120 make polar and hydrophobic interactions with the amino acid residues 372-379.
The interactions between the N and M domains lead to the stabilization of the closed state.
Active site
The constraint from the open state of Hsp90 to its closed state leads to the formation of the active site. Hydrogen bonding between the β-phosphate and the lid segment residues Gly 100 and Val 122 and hydrogen bonding between the γ-phosphate and the lid segment residues Gln 119, Gly 121, Val 122, Gly 123 and Arg 380 occurs due to the movement of the lid. Arg 380, which is part of the M domain, is the only residue that makes a contact wth the ATP outside the N domain. Glu 33 and Arg 380, which are in close proximity of ATP, are thought to play a highly important role in catalysis as their mutations leads to no catalytic activity. Asp 79 is also thought to play a role in the catalytic machinery.
Of note is the fact the interacting residues in the active sites as well as in the hydrophobic and polar interactions of the N and M domains are conserved in the Hsp90 homologs (Ali et al., 2006, Pearl et al., 2008).
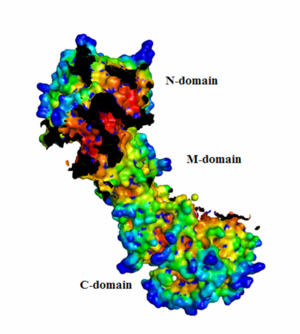
HotPatch analysis
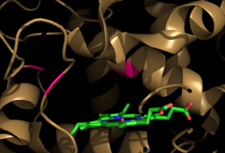
From the HotPatch analysis, it can be seen that that active site in the N-domain shows the most significant concave patch, which is colored as red. There is a lesser significant concave patch colored as red in the C-domain dimerization site too. There are no other patches in the protein that are as significant as the two patches mentioned above. In addition, there is a significant amount of data to show that the binding of ATP to Hsp90 occurs in the N-domain. The X-ray crystallization data for the closed conformation of Hsp90 validates the presence of the hot patch in C-domain, which is formed at the interface of the dimer.
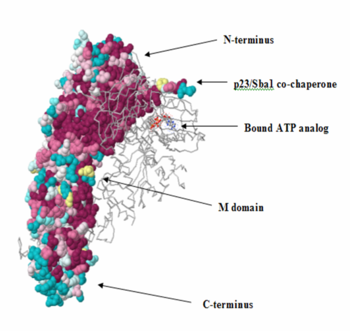
ConSurf analysis
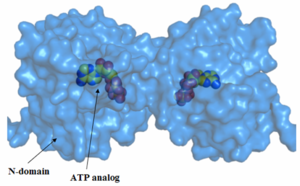
The N-terminus of the protein is the most conserved. The middle (M)-domain is less conserved as compared to the N-terminus and the C-terminus is the least conserved. These data make sense, because Hsp90 has ATPase activity. The ATPase activity is found in the N-terminus region and Hsp90 functions as an ATPase once ATP binds to the N-terminus region and two molecules of Hsp90 form a dimer. As a result, ATP is hydrolyzed and Hsp90 can carry out it function as a chaperone. The two arms that can be seen in the pictures are those of the p23/Sba1 co-chaperone, which make the activity of Hsp90 more efficient. It is known that Hsp90 has a molecular clamp mechanism driven by the binding of ATP at the N-terminus, which further validates the conservation of residues seen at the N-terminus and the more importantly, at the residues bound to the ATP analog in the molecule (shown in the pictures). The M-domain, which is functionally less active, shows conservation around the catalytic loop and the chains involved in the N-C interface. The C-terminus domain, the end for which only serves as the site for the formation of the dimerized molecular clamp, shows the least amount of conservation since its only function is to form the clamp and is not involved in the ATPase catalytic cycle. Thus, there is a decrease in the order of conservation from the N-terminus to the C-terminus.
There is a high level of conservation in the sites around the ATP binding region, which shows that the active site is conserved as also noted earlier.
Co-chaperone binding
Each p23/Sba1 co-chaperone molecule lies in a depression at the junction of the two N-domains. P23/Sba1 binding stabilizes the state required for client-protein activation. It binds to the closed state of Hsp90 70-fold more tightly than to the open state due to the correct conformation of the binding site for p23/Sba1. Additionally, p23/Sba1 shows no significant affinity for Hsp90 as a monomer. Thus, stabilization due to Hsp90 binding leads to the extension of the state of the chaperone that is required for client protein activation.
Possible mechanism
A proposed conformational cycle for Hsp90 is that the client protein can be transferred from the catalytic machinery of Hsp70 to the open form of Hsp90 by the simultaneous binding of another stabilization protein Hop to Hsp90 as well as Hsp70. Once bound, co-chaperones such as p23 can displace Hop, which can trigger conformational changes leading to the binding of p23 to Hsp90 and the subsequent stabilization of Hsp90. The ATP hydrolytic activity of Hsp90 can complete the cycle, which can be coupled to the activation and subsequent release of a client protein (Jackson et al., 2008).
Structures for Hsp90 homologs
Hsp90 is a ubiquitous protein and is conserved in many species. It shows a high amount of conservation in the N-terminal domain. Moreover, the active site, which is located in the N-terminal domain is highly conserved, which suggests a common mechanism for Hsp90. Also, there exists a dimerized state for HtpG, the bacterial homolog for Hsp90, and thus, there seems to be an ATPase coupled "molecular clamp" mechanism for Hsp90.
References
- Ali MMU, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH (2006) Crystal Structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature, 440: 1013-1018.
- Dollins DE, Warren JJ, Immormino RM, Gewirth DT, (2007) Structures of GRP94-nucleotide complexes reveal mechanistic difference between the Hsp90 chaperones. Molecular Cell, 1: 41-56.
- Jackson SE (2008) The solution to multiple structures. Structure, 16: 659-661.
- Pearl LH, Prodromou C, Workman P (2008) The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J., 410: 439-453.
- Prodromou C, Roe SM, Piper PW, Pearl LH (1997) A molecular clamp in the crystal structure of the N-terminal domain of the yeast Hsp90 chaperone. Nature Structural Biology, 4: 477-482.
- Siligardi G, Hu B, Panaretou B, Piper PW, Pearl LH, Prodromou C (2004) Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J. Bol. Chem. 279: 51989-51998.
- Vaughan CK, Piper PW, Pearl LH, Prodromou C (2008) A common conformationally coupled ATPase mechanism for yeast and human cytoplasmin HSP90s. FEBS Journal, 10:1-11.