User talk:Anders Lewisesquerre
From Proteopedia
Contents |
Brain-derived neurotrophic factor (BDNF)
Introduction
Brain-derived neurotrophic factor (BDNF) is a 13kDa signaling protein belonging to the neurotrophin family of growth factors. This family of growth factors are critical in the development, differentiation, and survival of neurons. Mammalian members of the neurotrophin family of growth factors include Nerve growth factor (NGF)
PDB structure, neurotrophin-3 (NT-3) PDB structure, and neurotrophin-4/5 (NT-4/5) PDB structure of neurotrophin-4/5.
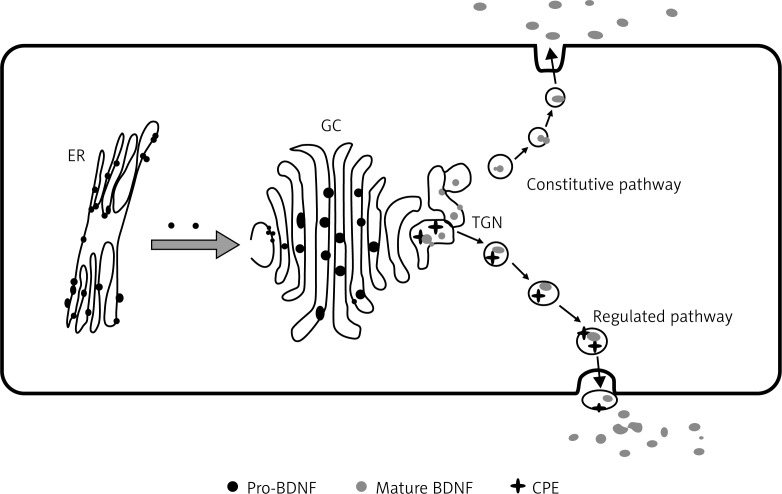
BDNF is synthesized in the Endoplasmic reticulum as a 32-35 kDa precursor (pro BDNF). This precursor is processed in the golgi-apparaturs and its terminal domain is cleaved by a convertase, the mature BDNF protein is then passed through the trans-golgi network and secreted by secretory vesicles out of the plasma membrane. BDNF is primarily expressed in the brain, where it is found in the hippocampus, cortex, amygdala, and striatum, and less so in the hypothalamus. BDNF is also expressed in peripheral tissues, such as the kidneys, retina, prostate, motor neurons, skeletal muscle.
Function
Brain-derived neurotrophic factor (BDNF) is involved in the growth, differentiation, and survival of neurons in the central, and peripheral nervous systems. It plays a role in neural plasticity, neurogenesis, energy metabolism, inflammation and immunity, neurotransmitter regulation, and potential roles in the cardiovascular system. The role of BDNF in neural plasticity is shown in its ability to regulate NDMA receptor signaling by increasing calcium influx and more BDNF release, which by retrograde signaling, enhances pre-synaptic vesicle cycling, thereby regulating long-term potentiation (LTP), and plasticity.
Mechanism
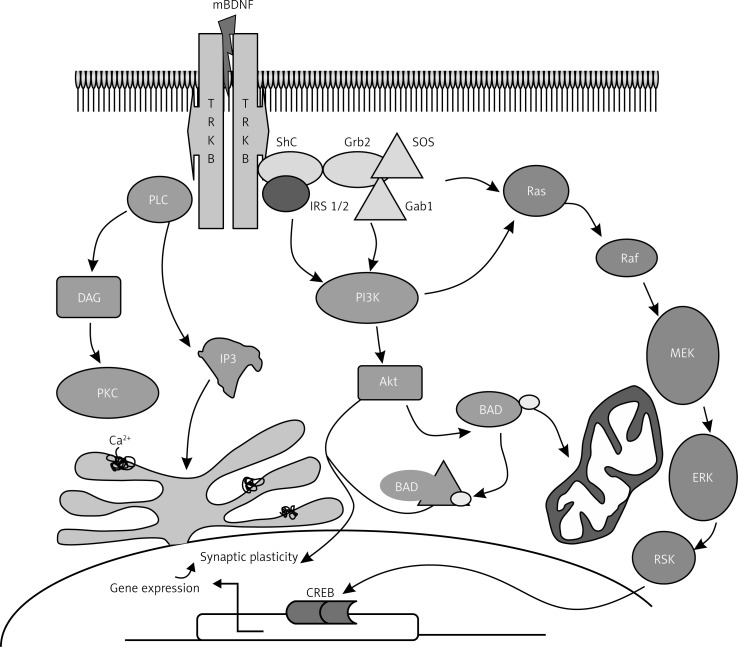
Effects of the mammalian neurotrophin family of proteins are accomplished through activation of the tropomyosin-related kinase receptor family (TrkA, TrkB, and TrkC), and the p75 neurotrophin receptor, which is a member of the tumor necrosis factor receptor superfamily. Binding to the p75 neurotrophin receptor leads to neurite outgrowth via Trk modulation, and activation of (JNK), which leads to apoptosis. As a reference, the complex formed upon binding of Nerve growth factor (NGF) – a neurotrophin with close structural homology to BDNF – to the p75 receptor is , where NGF is shown in lime, and the p75 receptor is shown in fuchsia. BDNF binds to (TrkB). A between a closely related neurotrophin (neurotrophin-4/5), was revealed via x-ray diffraction. Analysis of this interaction shows that key regions of the protein involved in direct with the receptor lie within the long, 𝛽-sheet regions of the protein as mentioned earlier, as well as flexible regions on the outer regions of neurotrophin-4/5 (although these regions are absent in BDNF. Receptor binding causes autophosphorylation of tyrosine residues and results in activation of three different signaling cascades. These signaling pathways cause activation of CREB and CREB-binding protein (CBP), transcription factors which regulate expression of genes involved in neural plasticity, cell survival, and stress resistance. The IRS-1/PI3K/AKT pathway Involves sequential activation of Insulin receptor substrate 1 (IRS1/2), phosphatidylinositol-3-kinase (PI3K), and protein kinase B (Akt). The pathway activates protein kinase A (Akt) which suppresses apoptosis In the Ras/MAPK/ERK pathway, Ras activates the Ras/MAPK/ERK pathway, as well as the PIK3, and PLC pathways. MAPK/ERK promotes cell survival by induction of pro survival genes and inhibition of pro-apoptotic proteins The third cascade is the PLC/DAG/IP3 pathway, where PLC-𝛾 is phosphorylated by BDNF binding to the TrkB receptor, leading to production of IP3 and DAG. IP3 increases intracellular calcium concentrations, and DAG regulates protein kinase C, which is important in the MAPK/ERK pathway.
Structure
|
Disease
Implicated in multiple neurodegenerative diseases due to its apparent neuroprotective effects, ability to promote neurogenesis, and improve synaptic plasticity. Lower BDNF levels are seen in patients with Multiple Sclerosis (MS), Parkinson's disease, and Alzheimer's disease, which highlights the consequence that lower amounts of BDNF contribute to the cognitive decline seen in Alzheimer’s, and dopaminergic neuron degeneration seen in Parkinson's. Huntington's disease, which is caused by a mutation in the huntingtin gene, produces a mutated huntingtin protein that disrupts BDNF axonal transport and transcription. Because striatal neurons heavily rely on cortically derived BDNF for survival and function, this disruption leads to the degeneration of striatal neurons, a key pathological feature of Huntington's disease. BDNF is also associated with type-2 diabetes mellitus by its regulation of energy metabolism, specifically through increasing the amount of insulin secreting granules in pancreatic 𝛃 cells through TrkB signaling pathways.
Val66Met polymorphism
A single nucleotide polymorphism has gained attention that involves a polymorphism at codon 66 in the BDNF gene that results in a point mutation swapping a valine for a methionine. While the mutation change does not make it into the final processed mature BDNF protein, it is known to induce impacts on the processing, transport, and potentially folding of the protein. Individuals with the met allele, especially homozygotes, have measurable effects on brain function caused by a decrease in the availability of BDNF at synapses as a result of the mutation. Individuals with this mutation may have decreased hippocampal volume and show symptoms of memory impairment.
Additional resources
References
Bathina, Siresha, and Undurti N Das. “Brain-derived neurotrophic factor and its clinical implications.” Archives of medical science : AMS vol. 11,6 (2015): 1164-78. doi:10.5114/aoms.2015.56342
https://www.uniprot.org/uniprotkb/P23560/entry
https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2019.00363/full
https://onlinelibrary.wiley.com/doi/10.1110/ps.8.12.2589
Skaper S. D. (2012). The neurotrophin family of neurotrophic factors: an overview. Methods in molecular biology (Clifton, N.J.), 846, 1–12. https://doi.org/10.1007/978-1-61779-536-7_1
Ibrahim, Abdallah Mohammad et al. “Brain-Derived Neurotropic Factor in Neurodegenerative Disorders.” Biomedicines vol. 10,5 1143. 16 May. 2022, doi:10.3390/biomedicines10051143