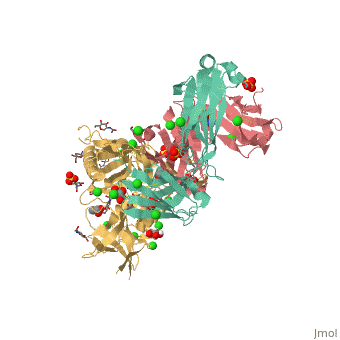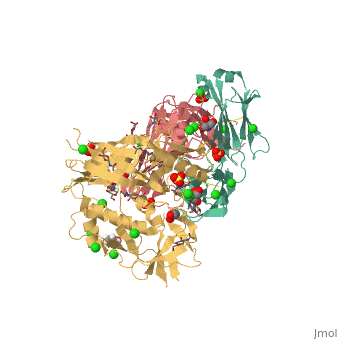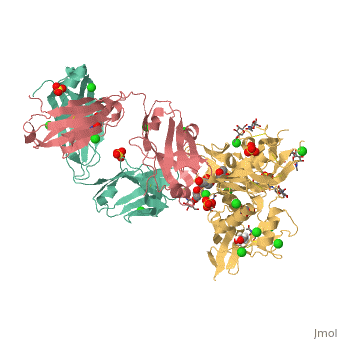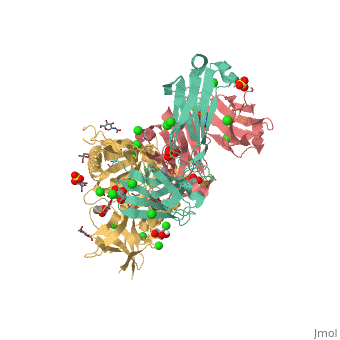
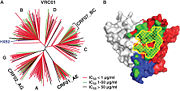
The crystal structure of VRC01 and VRC01-like antibodies are studied to define with characteristics are important in neutralizing HIV-1.
(3se9).
Introduction
HIV-1 has a high level of antigenic and genetic diversity. HIV-1 has also evolved mechanisms to evade the humoral immune response. These aspects of HIV-1 have made it difficult to develop a vaccine. After several years of infection, 10 to 25% of HIV-1 infected individuals develop neutralizing antibodies. Some antibodies target the transmembrane gp41 molecules of the HIV-1 viral spike, however most target the surface protein gp120[1]. VRC01 and VRC01-like antibodies bind to gp120 and are able to neutralize about 90% of HIV-1 isolates. Structural analysis has shown which characteristics of antibodies are essential to its binding with gp120[2]. Discovery of the structure of these antibodies can help develop an effective HIV-1 vaccine.
HIV-1 Neutralization
HIV-1 enters its host by binding viral gp120, a surface glycoprotein of HIV, to the host cell’s CD4 receptor. This interaction induces conformational changes in gp120[1]. This conformational change results in the exposure of a binding site for the co-receptor, usually CCR5 OR CXCR4[3]. The conformational changes also result in the formation of a pre-hairpin intermediate conformation in which gp41, a transmembrane glycoprotein of HIV, rearranges its molecules so that its N-terminal peptides form a trimer of helices that present a fusion peptide to the target cell. Once fusion occurs between the fusion peptide and the target cell membrane, HIV is able to enter and infect the target cell[4]. VRC01 binds to CD4’s binding site on gp120, preventing the CD4 receptor from binding to HIV and infecting the cell[1].
Structural Features
(3ngb).
Similarities to CD4 in complex with gp120. Analysis of VRC01 in complex with gp120 shows that this complex covers 98% of the CD4 binding site. However, VRC01’s binding site extends outside that of CD4, making it vulnerable to resistance of VRC01 neutralization by antigenic variation[5].
In Picture 1B, the contact surface of VRC01 and CD4 are shown on gp120. The green represents VRC01's contact surface and yellow represents CD4's contact surface[2]. Both the heavy chain and light chain of VRC01 contribute to the contact surfaces of the VRC01 gp120 complex. The focus of the binding is on the heavy chain second complementary-determining region. Over 50% of the surface contact involves the heavy chain second complementary-determining region; this is similar to CD4’s interaction with gp120. Two dominant residues, Phe43 and Arg59, are involved in CD4’s binding to gp120. Of these two residues, only the arginine interaction is mimicked by VRC01. This dominant interaction is between Asp368 of gp120 and Arg59 of the CD4 receptor and between Asp368 of gp120 and Arg71 of VRC01. Arg71 and Asp368 form a [5].
Similarities to other antibodies in complex with gp120. Although only about 50% of the amino acids in the variable region of the heavy chains of different CD4 binding site antibodies were conserved, the structures of each of their complex with gp120 was similar. Comparison between other CD4 binding site antibodies show that the Arg71 and Asp368 interaction is also conserved. From sequence analysis of 10 antibodies of the same IGHV1-2*02 germline, 70-90 nucleotide changes are made. Only two residues changes from this germline mature into the same amino acids. These changes occur in a hydrophobic contact of the heavy chain second complementary-determining region. The two amino acid changes are Gly56 into Ala56 and Thr57 into Val57[1]. More at this position led to an increased potency and breadth by increasing contacts with gp120’s bridging domain[6]. Another is the interaction of Tyr91 and Glu96 of VRC01 with loop D of gp120. These residues engage loop D by polar interactions[7].
Also, when 10 best antibody sequences were aligned, it was found that 68 heavy chain residues were conserved and 7 of these residues were involved in the contact between VRC01 and gp120. In comparison only 53 light chain residues were conserved and only 3 of these residues were involved in the contact between VRC01 and gp120. This is consistent with other research that has shown the light chain of VRC01 having a limited role in attachment to gp120 in comparison to the heavy chain[7].
Other features. VRC01 light chain residues, , make contacts with the protein-proximal N-acetyl-glucosamine from the N-linked glycan residue 276 of gp120. While other structures are blocked from binding because glycan shielding, VRC01 takes advantage of the glycan for binding[5].
HIV Prevention Research
In 2003, Veazey and fellow researchers found that early broadly neutralizing antibodies had microbicide potential by using a monkey cell as the model. The microbicide used on these monkeys consisted of b12, a broadly neutralizing antibody. These monkeys were challenged with SHIV, simian-human immunodeficiency virus, through the vagina. Only three of the twelve monkeys became infected. It was also found that the protection against HIV lasts for up to two hours[8]. These results show that microbicides containing antibodies are effective at preventing HIV in monkeys.
A similar experiment was done in 2012; it used humanized mouse models called RAG-hu mice, which contained human target cells. Results show that seven out of nine mice that were administered the VRC01 antibody and all mice that were given a cocktail containing four broadly neutralizing antibodies as a topical gel were protected against HIV-1. These results showed that broadly neutralizing antibodies could be used as a topical microbicide to prevent vaginal transmission of HIV and that a combination of antibodies can provide better protection against HIV. When the VRC01 antibody and the broadly neutralizing antibody cocktail were administered to the humanized mice via the intravenous route, none of the mice were infected with SHIV[9].