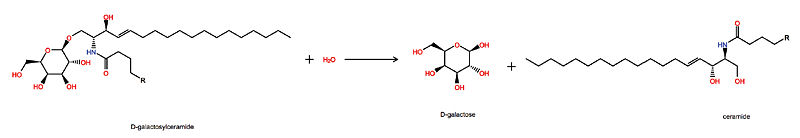
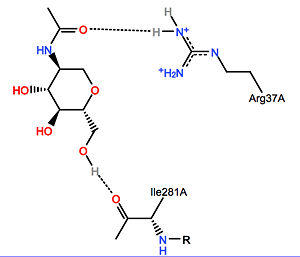
Galactosylceramidase
From Proteopedia
| Line 47: | Line 47: | ||
*calcium ion | *calcium ion | ||
*N-acetyl-D-glucosamine | *N-acetyl-D-glucosamine | ||
| - | [[Image:NAG-galactosylceramidase.jpg|center|300px|NAG-galactosylceramidase ligand-enzyme interaction]] | + | [[Image:NAG-galactosylceramidase.jpg|thumb|center|300px|NAG-galactosylceramidase ligand-enzyme interaction]] |
| + | |||
==Inhibitors== | ==Inhibitors== | ||
Inhibitory molecules in humans include: | Inhibitory molecules in humans include: | ||
Revision as of 04:49, 4 June 2014
| |||||||||||
Krabbe disease is a devastating neurodegenerative disease characterized by widespread demyelination that is caused by defects in the enzyme galactocerebrosidase (GALC). Disease-causing mutations have been identified throughout the GALC gene. However, a molecular understanding of the effect of these mutations has been hampered by the lack of structural data for this enzyme. Here we present the crystal structures of GALC and the GALC-product complex, revealing a novel domain architecture with a previously uncharacterized lectin domain not observed in other hydrolases. All three domains of GALC contribute residues to the substrate-binding pocket, and disease-causing mutations are widely distributed throughout the protein. Our structures provide an essential insight into the diverse effects of pathogenic mutations on GALC function in human Krabbe variants and a compelling explanation for the severity of many mutations associated with fatal infantile disease. The localization of disease-associated mutations in the structure of GALC will facilitate identification of those patients that would be responsive to pharmacological chaperone therapies. Furthermore, our structure provides the atomic framework for the design of such drugs.
Insights into Krabbe disease from structures of galactocerebrosidase., Deane JE, Graham SC, Kim NN, Stein PE, McNair R, Cachon-Gonzalez MB, Cox TM, Read RJ, Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15169-73. Epub 2011 Aug 29. PMID:21876145
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Ligands
- calcium ion
- N-acetyl-D-glucosamine
Inhibitors
Inhibitory molecules in humans include:
- 6-hexadecanoylamino-4-methylbelliferyl-beta-D-galactopyranoside, competitive inhibition
- D-galactose
- galactonyl hydrazide
- lactose
- N-(6-aminohexyl)-D-galactoside
- taurocholate (at high concentrations above 0.3% w/v)[7]
References
- ↑ RCSB Protein Data Bank - RCSB PDB - 3ZR5 Structure Summary. (n.d.). RCSB Protein Data Bank - RCSB PDB - 3ZR5 Structure Summary. Retrieved June 3, 2014, from www.rcsb.org DOI:10.2210/pdb3zr5/pdb
- ↑ Zizioli D, Guarienti M, Tobia C, Gariano G, Borsani G, Bresciani R, Ronca R, Giacopuzzi E, Preti A, Gaudenzi G, Belleri M, Di Salle E, Fabrias G, Casas J, Ribatti D, Monti E, Presta M. Molecular cloning and knockdown of galactocerebrosidase in zebrafish: new insights into the pathogenesis of Krabbe's disease. Biochim Biophys Acta. 2014 Apr;1842(4):665-75. doi: 10.1016/j.bbadis.2014.01.008., Epub 2014 Jan 24. PMID:24463171 doi:http://dx.doi.org/10.1016/j.bbadis.2014.01.008
- ↑ 3.0 3.1 Deane JE, Graham SC, Kim NN, Stein PE, McNair R, Cachon-Gonzalez MB, Cox TM, Read RJ. Insights into Krabbe disease from structures of galactocerebrosidase. Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15169-73. Epub 2011 Aug 29. PMID:21876145 doi:10.1073/pnas.1105639108
- ↑ Visigalli I, Ungari S, Martino S, Park H, Cesani M, Gentner B, Sergi Sergi L, Orlacchio A, Naldini L, Biffi A. The galactocerebrosidase enzyme contributes to the maintenance of a functional hematopoietic stem cell niche. Blood. 2010 Sep 16;116(11):1857-66. doi: 10.1182/blood-2009-12-256461. Epub 2010 , May 28. PMID:20511539 doi:http://dx.doi.org/10.1182/blood-2009-12-256461
- ↑ 5.0 5.1 Belleri M, Ronca R, Coltrini D, Nico B, Ribatti D, Poliani PL, Giacomini A, Alessi P, Marchesini S, Santos MB, Bongarzone ER, Presta M. Inhibition of angiogenesis by beta-galactosylceramidase deficiency in globoid cell leukodystrophy. Brain. 2013 Sep;136(Pt 9):2859-75. doi: 10.1093/brain/awt215. PMID:23983033 doi:http://dx.doi.org/10.1093/brain/awt215
- ↑ 6.0 6.1 6.2 Kohlschutter A. Lysosomal leukodystrophies: Krabbe disease and metachromatic leukodystrophy. Handb Clin Neurol. 2013;113:1611-8. doi: 10.1016/B978-0-444-59565-2.00029-0. PMID:23622382 doi:http://dx.doi.org/10.1016/B978-0-444-59565-2.00029-0
- ↑ EC 3.2.1.46 - galactosylceramidase. (n.d.). Information on. Retrieved June 3, 2014, from www.brenda-enzymes.org
Proteopedia Page Contributors and Editors (what is this?)
Alison Stivers, Michal Harel, Dillon Shapiro, Angel Herraez, Joel L. Sussman