Chaperonin
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
The most characterized CPN are in the GroEL/GroES complex from ''Escherichia coli'' and CPN60/CPN10 from ''Thermus thermophilus''. The larger subunit (GroEL, CPN60) contains 3 domains. The apical domain is the one which binds the substrate. Group II CPNs are found in eukaryotic cytosol and archaea. Thermosome is a CPN complex found in archaea. CCT is a CPN complex found in eukarya. | The most characterized CPN are in the GroEL/GroES complex from ''Escherichia coli'' and CPN60/CPN10 from ''Thermus thermophilus''. The larger subunit (GroEL, CPN60) contains 3 domains. The apical domain is the one which binds the substrate. Group II CPNs are found in eukaryotic cytosol and archaea. Thermosome is a CPN complex found in archaea. CCT is a CPN complex found in eukarya. | ||
| + | See also [[Chaperones]]. | ||
== 3D Structures of Chaperonin == | == 3D Structures of Chaperonin == | ||
Revision as of 12:11, 24 December 2014
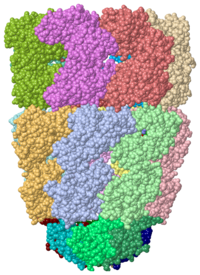
Crystal Structure of Chaperonin, 1pcq
Chaperonins (CPN) are oligomeric proteins that mediate the folding of polypeptide chains. Group I CPN are found in bacteria, chloroplasts and mitochondria. For an introductory overview, see Chaperonins in Wikipedia.
The most characterized CPN are in the GroEL/GroES complex from Escherichia coli and CPN60/CPN10 from Thermus thermophilus. The larger subunit (GroEL, CPN60) contains 3 domains. The apical domain is the one which binds the substrate. Group II CPNs are found in eukaryotic cytosol and archaea. Thermosome is a CPN complex found in archaea. CCT is a CPN complex found in eukarya. See also Chaperones.
3D Structures of Chaperonin
Updated on 24-December-2014
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Eric Martz, Jaime Prilusky
