This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Wade Cook/Sandbox 1
From Proteopedia
| Line 9: | Line 9: | ||
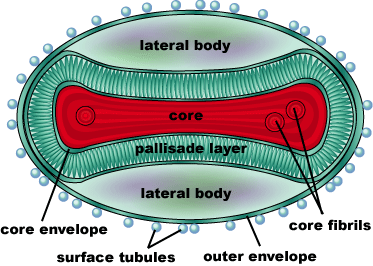
The smallpox virus invades host cells by binding to specific receptors on the host cell membrane. The virus binds to host cell receptors via hemagglutinin antigens expressed on its outer surface. The exact mechanism of entry is not yet known. Upon invasion of a host cell, the smallpox virus replicates in the host cytoplasm, rather than the host nucleus, using many of it’s own enzymes to replicate. Replication begins once the virion has reached the cytoplasm of the host cell. First, a messenger RNA molecule is transcribed by RNA polymerase and related enzymes before the genome is uncoated. The first genes that are transcribed, known as early genes, code for proteins that facilitate the uncoating of the viral genome and initiate a second round of transcription of intermediate genes. The intermediate genes produce mRNA which are translated into proteins that allow the transcription of the late class of genes. The late class genes encode proteins which make up the structural and enzymatic components of a new virion. Further replication of this virus leads to the eventual shutdown of the host cell’s DNA, RNA, and protein synthesis, and causes changes to the cell’s architecture to allow the virus to use the host cell’s genetic machinery for reproduction. | The smallpox virus invades host cells by binding to specific receptors on the host cell membrane. The virus binds to host cell receptors via hemagglutinin antigens expressed on its outer surface. The exact mechanism of entry is not yet known. Upon invasion of a host cell, the smallpox virus replicates in the host cytoplasm, rather than the host nucleus, using many of it’s own enzymes to replicate. Replication begins once the virion has reached the cytoplasm of the host cell. First, a messenger RNA molecule is transcribed by RNA polymerase and related enzymes before the genome is uncoated. The first genes that are transcribed, known as early genes, code for proteins that facilitate the uncoating of the viral genome and initiate a second round of transcription of intermediate genes. The intermediate genes produce mRNA which are translated into proteins that allow the transcription of the late class of genes. The late class genes encode proteins which make up the structural and enzymatic components of a new virion. Further replication of this virus leads to the eventual shutdown of the host cell’s DNA, RNA, and protein synthesis, and causes changes to the cell’s architecture to allow the virus to use the host cell’s genetic machinery for reproduction. | ||
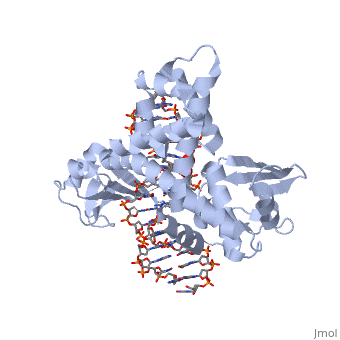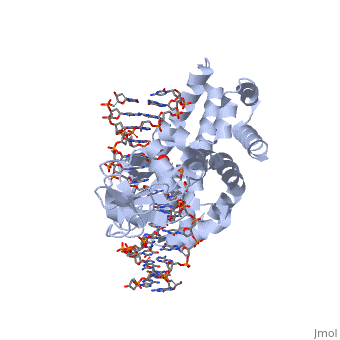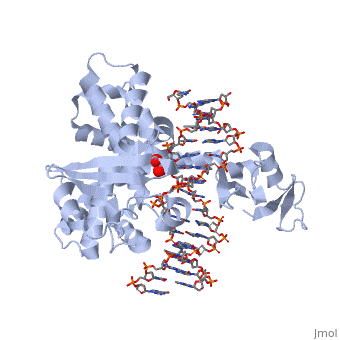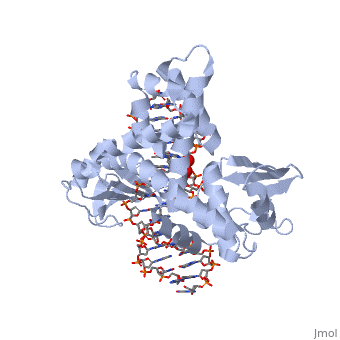
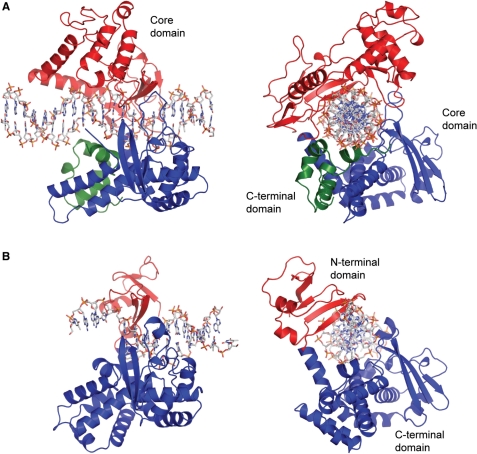
One characteristic that makes the Variola virus so deadly is that it doesn’t require the host cell’s genetic machinery to begin replication. The reason for this is that the Variola virus codes for all the enzymes needed for its proliferation. One enzyme in particular, the type IB topoisomerase is responsible for unwinding the packaged, supercoiled, viral DNA to initiate viral replication. This is accomplished through a nick-joining mechanism of the double stranded DNA, in which a tyrosine nucleophile attacks a phosphodiester bond to form a 3’ phosphotyrosine linkage and releases a free 5’ hydroxyl group. This creates a nick to unwind supercoiling, which is then re-ligated to form relaxed DNA. Most mechanisms involving DNA including replication, transcription, and repair induce a certain degree of positive supercoiling. Tension due to the positive supercoiling buildup can be relieved by the enzymes topoisomerase. The variola topoisomerase 1B is an unusual topoisomerase. It is by far the smallest topoisomerase (33Kda) and only acts at specific sites that contain the sequence 5’-(T/C)CCTT-3’ in the variola genome. The topoisomerase 1B is made up of two domains that wrap around this recognition sequence forming a clamp around the DNA. The smaller amino terminal domain is responsible for interactions with the DNA sequence, whereas the larger C-terminal domain contains the active site and is the area responsible for all enzymatic activity. | One characteristic that makes the Variola virus so deadly is that it doesn’t require the host cell’s genetic machinery to begin replication. The reason for this is that the Variola virus codes for all the enzymes needed for its proliferation. One enzyme in particular, the type IB topoisomerase is responsible for unwinding the packaged, supercoiled, viral DNA to initiate viral replication. This is accomplished through a nick-joining mechanism of the double stranded DNA, in which a tyrosine nucleophile attacks a phosphodiester bond to form a 3’ phosphotyrosine linkage and releases a free 5’ hydroxyl group. This creates a nick to unwind supercoiling, which is then re-ligated to form relaxed DNA. Most mechanisms involving DNA including replication, transcription, and repair induce a certain degree of positive supercoiling. Tension due to the positive supercoiling buildup can be relieved by the enzymes topoisomerase. The variola topoisomerase 1B is an unusual topoisomerase. It is by far the smallest topoisomerase (33Kda) and only acts at specific sites that contain the sequence 5’-(T/C)CCTT-3’ in the variola genome. The topoisomerase 1B is made up of two domains that wrap around this recognition sequence forming a clamp around the DNA. The smaller amino terminal domain is responsible for interactions with the DNA sequence, whereas the larger C-terminal domain contains the active site and is the area responsible for all enzymatic activity. | ||
| - | [[Image:Comparison_of_Euk_and_Viral_Topo.jpg]] | + | [[Image:Comparison_of_Euk_and_Viral_Topo.jpg ]] |
The type 1B topoisomerase can relieve negative or positive supercoiling without the use of ATP. As long as there's torsional strain on the DNA strand from the supercoiling, this is enough potential energy to drive the uncoiling of the strand. Both eukaryotic and viral topoisomerase 1B enzymes contain a highly conserved active site consisting of five common amino acid residues. These residues are Tyr, Arg, Arg, Lys, His/Asp (figure 5). Researchers have proven that an asparagine residue can replace the histidine residue in the active site of both eukaryotic and viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s.Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs. | The type 1B topoisomerase can relieve negative or positive supercoiling without the use of ATP. As long as there's torsional strain on the DNA strand from the supercoiling, this is enough potential energy to drive the uncoiling of the strand. Both eukaryotic and viral topoisomerase 1B enzymes contain a highly conserved active site consisting of five common amino acid residues. These residues are Tyr, Arg, Arg, Lys, His/Asp (figure 5). Researchers have proven that an asparagine residue can replace the histidine residue in the active site of both eukaryotic and viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s.Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs. | ||
Revision as of 15:40, 16 November 2015
Smallpox (Variola Virus) - Topoisomerase 1B
| |||||||||||
References
Berwald, Juli. "Variola Virus." Encyclopedia of Espionage, Intelligence, and Security. 2004.Encyclopedia.com. 28 Oct. 2015 <http://www.encyclopedia.com>.
"PENN Medicine News: Penn Researchers Determine Structure of Smallpox Virus Protein Bound to DNA." PENN Medicine News: Penn Researchers Determine Structure of Smallpox Virus Protein Bound to DNA. PENN Medicine, 4 Aug. 2006. Web. 28 Oct. 2015. <http://www.uphs.upenn.edu/news/News_Releases/aug06/smlpxenz.htm>.
Perry, Kay, Young Hwang, Frederic D. Bushman, and Gregory D. Van Duyne. "Insights from the Structure of a Smallpox Virus Topoisomerase-DNA Transition State Mimic." Structure (London, England : 1993). U.S. National Library of Medicine, n.d. Web. 28 Oct. 2015. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822398/>.
“Poxvirus.” Encyclopaedia Britannica. Britannica Academic. Encyclopædia Britannica Inc., 2015. Web. 28 Oct. 2015. <http://academic.eb.com/EBchecked/topic/473442/poxvirus>.
Shubhash, and Parija. "Poxviruses." Textbook of Microbiology and Immunity. Ed. Chandra. India: Elsevior, 2009. 484. Print.
“Smallpox.” Center for Disease Control and Prevention. CDC, n.d. Web. 28 Oct. 2015. <http://www.bt.cdc.gov/agent/smallpox/index.asp>.