Chaperonin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
[[Image:1pcq.png|left|200px|thumb|Crystal Structure of Chaperonin, [[1pcq]]]] | [[Image:1pcq.png|left|200px|thumb|Crystal Structure of Chaperonin, [[1pcq]]]] | ||
| - | {{STRUCTURE_1pcq| PDB=1pcq | SIZE=400| SCENE=Chaperonin/Groel_groes_comnplex/1 |right|CAPTION=GroEL/GroES complex with ADP, AlF3, Mg+2 and K+ ions, [[1pcq]] }} | ||
| - | <StructureSection load='1pcq' size='350' side='right' caption='GroEL/GroES complex with ADP, AlF3, Mg+2 and K+ ions (PDB entry [[1pcq]])' scene=''> | + | <StructureSection load='1pcq' size='350' side='right' caption='GroEL/GroES complex with ADP, AlF3, Mg+2 and K+ ions (PDB entry [[1pcq]])' scene='Chaperonin/Groel_groes_comnplex/1'> |
'''Chaperonins''' (CPN) are oligomeric proteins that mediate the folding of polypeptide chains. Group I CPN are found in bacteria, chloroplasts and mitochondria. For an introductory overview, see [http://en.wikipedia.org/wiki/Chaperonins Chaperonins in Wikipedia]. | '''Chaperonins''' (CPN) are oligomeric proteins that mediate the folding of polypeptide chains. Group I CPN are found in bacteria, chloroplasts and mitochondria. For an introductory overview, see [http://en.wikipedia.org/wiki/Chaperonins Chaperonins in Wikipedia]. | ||
Revision as of 11:30, 6 December 2015
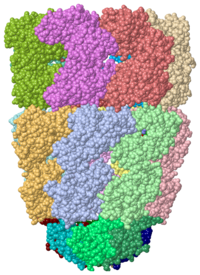
Crystal Structure of Chaperonin, 1pcq
| |||||||||||
3D Structures of Chaperonin
Updated on 06-December-2015
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Eric Martz, Jaime Prilusky
