Sandbox Wabash13
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
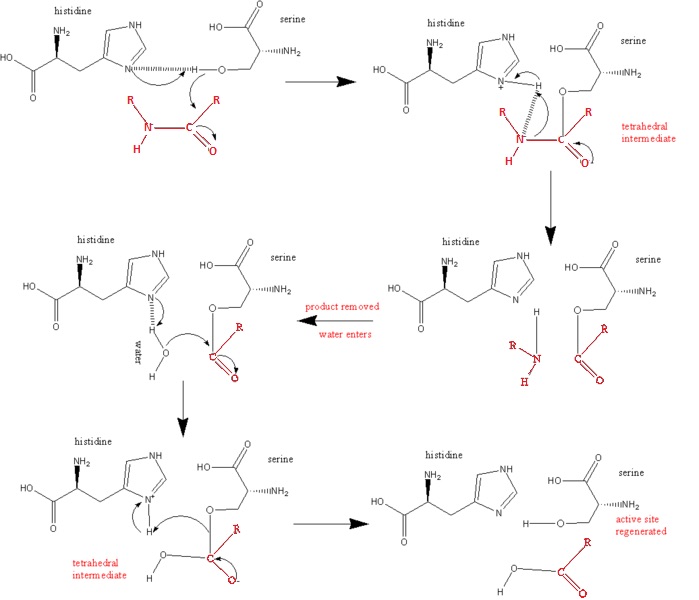
The nucleophilic attack is carried out by Ser 195, by attacking the scissile peptide's carbonyl group to form the tetrahedral intermediate. | The nucleophilic attack is carried out by Ser 195, by attacking the scissile peptide's carbonyl group to form the tetrahedral intermediate. | ||
| - | 2. Acid catalysis breaks the tetrahedral intermediate through cleaving of the scissile peptide bond to form an acyl-enzyme intermediate. His 57 donates a proton by general acid catalysis. This is aided by Asp 102 polarizing effect on His 57. This causes the tetrahedral intermediate to decompose to the acyl-enzyme intermediate. | + | 2. Acid catalysis breaks the tetrahedral intermediate through cleaving of the scissile peptide bond to form an acyl-enzyme intermediate. His 57 donates a proton by general acid catalysis. |
| + | This is aided by Asp 102 polarizing effect on His 57. This causes the tetrahedral intermediate to decompose to the acyl-enzyme intermediate. | ||
3. The amine product is replaced by H2O and subsequently released from the enzyme/substrate complex. | 3. The amine product is replaced by H2O and subsequently released from the enzyme/substrate complex. | ||
Revision as of 01:27, 19 February 2016
Trypsin Mechanism & Structure - Chase Francoeur, Elias Arellano
| |||||||||||