Journal:Molecular Cell:1
From Proteopedia
(Difference between revisions)

| Line 12: | Line 12: | ||
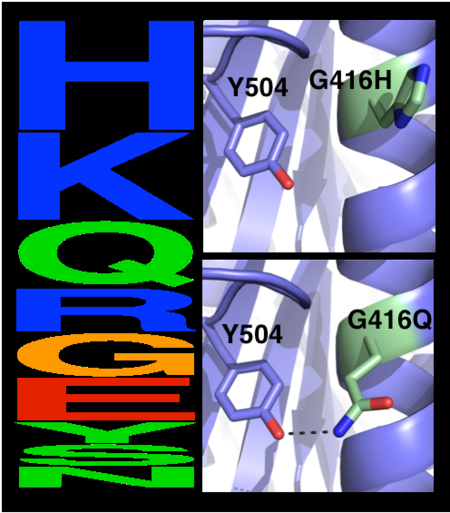
[[Image:MC1.png|left|450px|thumb|Eliminating potentially destabilizing mutations through homologous-sequence analysis and computational mutation scanning. Left: Sequence logo for hAChE position Gly416. The height of letters represents the respective amino acid’s frequency in an alignment of homologous AChE sequences. The evolutionarily ‘allowed’ sequence space (PSSM scores ≥0) at position 416 includes the 9 amino acids shown. Right: Structural models of mutations to the evolutionarily favored amino acid His, and to Gln, which is favored by Rosetta energy calculations. The His side chain is strained due to its proximity to the bulky Tyr504 aromatic ring, whereas the Gln side chain is relaxed and forms a favorable hydrogen bond with Tyr504 (dashed line)]] | [[Image:MC1.png|left|450px|thumb|Eliminating potentially destabilizing mutations through homologous-sequence analysis and computational mutation scanning. Left: Sequence logo for hAChE position Gly416. The height of letters represents the respective amino acid’s frequency in an alignment of homologous AChE sequences. The evolutionarily ‘allowed’ sequence space (PSSM scores ≥0) at position 416 includes the 9 amino acids shown. Right: Structural models of mutations to the evolutionarily favored amino acid His, and to Gln, which is favored by Rosetta energy calculations. The His side chain is strained due to its proximity to the bulky Tyr504 aromatic ring, whereas the Gln side chain is relaxed and forms a favorable hydrogen bond with Tyr504 (dashed line)]] | ||
{{Clear}} | {{Clear}} | ||
| - | Scenes highlight stabilizing effects of <scene name='72/728277/Cv/10'>selected mutations</scene> (in red): | + | Scenes highlight stabilizing effects of <scene name='72/728277/Cv/10'>selected mutations</scene> (in <font color='red'><b>red</b></font>): |
*<scene name='72/728277/Cv/4'>Buried hydrogen bonds</scene>. | *<scene name='72/728277/Cv/4'>Buried hydrogen bonds</scene>. | ||
*<scene name='72/728277/Cv/5'>Surface polarity</scene>. | *<scene name='72/728277/Cv/5'>Surface polarity</scene>. | ||
*<scene name='72/728277/Cv/6'>Helix capping</scene>. | *<scene name='72/728277/Cv/6'>Helix capping</scene>. | ||
*<scene name='72/728277/Cv/7'>Loop rigidity</scene>. | *<scene name='72/728277/Cv/7'>Loop rigidity</scene>. | ||
| - | *<scene name='72/728277/Cv/8'>Core packing</scene>. <span style="color:cyan;background-color:black;font-weight:bold;">Wild type hAChE is shown in cyan</span> and <span style="color:lime;background-color:black;font-weight:bold;"> | + | *<scene name='72/728277/Cv/8'>Core packing</scene>. <span style="color:cyan;background-color:black;font-weight:bold;">Wild type hAChE is shown in cyan</span> and <span style="color:lime;background-color:black;font-weight:bold;">designed hAChE is in green</span>. |
| Line 26: | Line 26: | ||
*<scene name='72/728277/Cv/14'>Overall aligment</scene>. | *<scene name='72/728277/Cv/14'>Overall aligment</scene>. | ||
| - | *<scene name='72/728277/Cv/13'>Sub-Ångstrom accuracy in design of 2 small-to-large core mutations</scene> <span style="color:violet;background-color:black;font-weight:bold;" | + | *<scene name='72/728277/Cv/13'>Sub-Ångstrom accuracy in design of 2 small-to-large core mutations</scene> <span style="color:violet;background-color:black;font-weight:bold;">. |
| - | *<scene name='72/728277/Cv/ | + | *<scene name='72/728277/Cv/19'>The maximal deviation observed between any respective Cα atoms</scene> in the model and structure is 3.1 Å (dashed line). This conformation change likely results from elimination of a side chain-backbone hydrogen bond between Thr112 and Ser110 due to the designed Thr112Ala mutation. |
*<scene name='72/728277/Cv/16'>Comparison of designed buried hydrogen bonds</scene>. Val331Asn was predicted to form a hydrogen bond with Glu450 and another with Pro446 in the designed model; in the crystal structure, instead, Asn331 interacts with Glu334 and Glu450. | *<scene name='72/728277/Cv/16'>Comparison of designed buried hydrogen bonds</scene>. Val331Asn was predicted to form a hydrogen bond with Glu450 and another with Pro446 in the designed model; in the crystal structure, instead, Asn331 interacts with Glu334 and Glu450. | ||
*<scene name='72/728277/Cv/18'>Leu394Asn forms 2 hydrogen bonds with Pro388 and Asp390, as designed</scene>. | *<scene name='72/728277/Cv/18'>Leu394Asn forms 2 hydrogen bonds with Pro388 and Asp390, as designed</scene>. | ||
Revision as of 07:05, 3 May 2016
| |||||||||||
- ↑ REF
Proteopedia Page Contributors and Editors (what is this?)
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.