CRISPR-Cas9
From Proteopedia
(Difference between revisions)
| Line 63: | Line 63: | ||
The SaCas9 sgRNA consists of the guide region (G1–C20),repeat region (G21–G34), tetraloop (G35–A38), anti-repeat region (C39–C54), stem loop 1 (A56–G68), and single-stranded linker (U69–U73), with A55 connecting the anti-repeat region and stem loop 1. U73 at the 3' end is disordered in the present structure. The guide region (G1–C20) and the target DNA strand (dG1–dC20) form the <scene name='74/742625/Cv6/11'>guide:target heteroduplex</scene>, whereas the target DNA strand (dC(8)–dA(1)) and the non-target DNA strand (dT1*–dG8*) form a <scene name='74/742625/Cv6/12'>PAM-containing duplex</scene> (referred to as the PAM duplex). The repeat (G21–G34) and anti-repeat (C39–C54) regions form a distorted duplex (referred to as the <scene name='74/742625/Cv6/13'>repeat:anti-repeat duplex</scene>) via 13 Watson-Crick base pairs. <scene name='74/742625/Cv6/16'>Stem loop 1</scene> is formed via three Watson-Crick base pairs (G57:C67–C59:G65) and two non-canonical base pairs (A56:G68 and A60:A63). U64 does not base pair with A60 and is flipped out of the stem loop. The N1 and N6 of A63 hydrogen bond with the 2'-OH and N3 of A60, respectively. G68 stacks with G57:C67, with the G68 N2 interacting with the backbone phosphate group between A55 and A56. A55 adopts the ''syn'' conformation, and its adenine base stacks with U69. In addition, the N1 of A55 hydrogen bonds with the 2'-OH of G68, thus stabilizing the basal region of stem loop 1. An adenosine residue immediately after the repeat:anti-repeat duplex is highly conserved among CRISPR-Cas9 systems, and the equivalent adenosine in the SpCas9 sgRNA, A51, also adopts the ''syn'' conformation, suggesting that these adenosine residues play conserved key roles in connecting the repeat:anti-repeat duplex and stem loop 1. | The SaCas9 sgRNA consists of the guide region (G1–C20),repeat region (G21–G34), tetraloop (G35–A38), anti-repeat region (C39–C54), stem loop 1 (A56–G68), and single-stranded linker (U69–U73), with A55 connecting the anti-repeat region and stem loop 1. U73 at the 3' end is disordered in the present structure. The guide region (G1–C20) and the target DNA strand (dG1–dC20) form the <scene name='74/742625/Cv6/11'>guide:target heteroduplex</scene>, whereas the target DNA strand (dC(8)–dA(1)) and the non-target DNA strand (dT1*–dG8*) form a <scene name='74/742625/Cv6/12'>PAM-containing duplex</scene> (referred to as the PAM duplex). The repeat (G21–G34) and anti-repeat (C39–C54) regions form a distorted duplex (referred to as the <scene name='74/742625/Cv6/13'>repeat:anti-repeat duplex</scene>) via 13 Watson-Crick base pairs. <scene name='74/742625/Cv6/16'>Stem loop 1</scene> is formed via three Watson-Crick base pairs (G57:C67–C59:G65) and two non-canonical base pairs (A56:G68 and A60:A63). U64 does not base pair with A60 and is flipped out of the stem loop. The N1 and N6 of A63 hydrogen bond with the 2'-OH and N3 of A60, respectively. G68 stacks with G57:C67, with the G68 N2 interacting with the backbone phosphate group between A55 and A56. A55 adopts the ''syn'' conformation, and its adenine base stacks with U69. In addition, the N1 of A55 hydrogen bonds with the 2'-OH of G68, thus stabilizing the basal region of stem loop 1. An adenosine residue immediately after the repeat:anti-repeat duplex is highly conserved among CRISPR-Cas9 systems, and the equivalent adenosine in the SpCas9 sgRNA, A51, also adopts the ''syn'' conformation, suggesting that these adenosine residues play conserved key roles in connecting the repeat:anti-repeat duplex and stem loop 1. | ||
| + | |||
| + | '''Recognition Mechanism of the Guide:Target Heteroduplex''' | ||
| + | |||
| + | The <scene name='74/742625/Cv6/17'>guide:target heteroduplex is accommodated in the central channel</scene> formed between the REC and NUC lobes. The sugar-phosphate backbone of the PAM-distal region (A3–U6) of the sgRNA interacts with the REC lobe (Thr238, Tyr239, Lys248, Tyr256, Arg314, Asn394, and Gln414). | ||
=See aslo= | =See aslo= | ||
*[[Cas9]] | *[[Cas9]] | ||
Revision as of 11:25, 20 August 2018
| |||||||||||
References
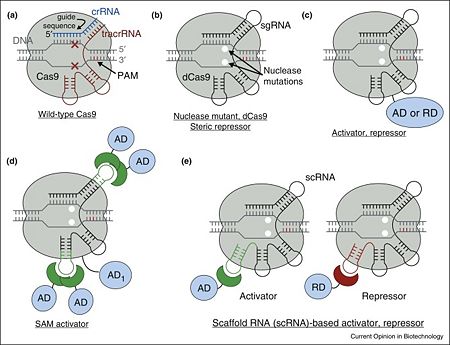
- ↑ 1.0 1.1 1.2 1.3 1.4 Didovyk A, Borek B, Tsimring L, Hasty J. Transcriptional regulation with CRISPR-Cas9: principles, advances, and applications. Curr Opin Biotechnol. 2016 Aug;40:177-84. doi: 10.1016/j.copbio.2016.06.003. Epub, 2016 Jun 23. PMID:27344519 doi:http://dx.doi.org/10.1016/j.copbio.2016.06.003
- ↑ Brophy JA, Voigt CA. Principles of genetic circuit design. Nat Methods. 2014 May;11(5):508-20. doi: 10.1038/nmeth.2926. PMID:24781324 doi:http://dx.doi.org/10.1038/nmeth.2926
- ↑ Straubeta A, Lahaye T. Zinc fingers, TAL effectors, or Cas9-based DNA binding proteins: what's best for targeting desired genome loci? Mol Plant. 2013 Sep;6(5):1384-7. doi: 10.1093/mp/sst075. Epub 2013 May 29. PMID:23718948 doi:http://dx.doi.org/10.1093/mp/sst075
- ↑ Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014 Apr;32(4):347-55. doi: 10.1038/nbt.2842. Epub 2014 Mar 2. PMID:24584096 doi:http://dx.doi.org/10.1038/nbt.2842
- ↑ Marraffini LA. CRISPR-Cas immunity in prokaryotes. Nature. 2015 Oct 1;526(7571):55-61. doi: 10.1038/nature15386. PMID:26432244 doi:http://dx.doi.org/10.1038/nature15386
- ↑ Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJ, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015 Nov;13(11):722-36. doi: 10.1038/nrmicro3569. Epub 2015, Sep 28. PMID:26411297 doi:http://dx.doi.org/10.1038/nrmicro3569
- ↑ 7.0 7.1 7.2 7.3 Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015 Jun 26;348(6242):1477-81. doi: 10.1126/science.aab1452. PMID:26113724 doi:http://dx.doi.org/10.1126/science.aab1452
- ↑ 8.0 8.1 8.2 8.3 8.4 Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816-21. doi: 10.1126/science.1225829. Epub 2012, Jun 28. PMID:22745249 doi:http://dx.doi.org/10.1126/science.1225829
- ↑ 9.0 9.1 9.2 Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):E2579-86. Epub 2012 Sep 4. PMID:22949671 doi:http://dx.doi.org/10.1073/pnas.1208507109
- ↑ 10.0 10.1 10.2 10.3 Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013 Feb 28;152(5):1173-83. doi: 10.1016/j.cell.2013.02.022. PMID:23452860 doi:http://dx.doi.org/10.1016/j.cell.2013.02.022
- ↑ 11.0 11.1 11.2 Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013 Aug;41(15):7429-37. doi: 10.1093/nar/gkt520. Epub 2013, Jun 12. PMID:23761437 doi:http://dx.doi.org/10.1093/nar/gkt520
- ↑ Kuscu C, Arslan S, Singh R, Thorpe J, Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol. 2014 Jul;32(7):677-83. doi: 10.1038/nbt.2916. Epub 2014 May 18. PMID:24837660 doi:http://dx.doi.org/10.1038/nbt.2916
- ↑ Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA. Structures of Cas9 Endonucleases Reveal RNA-Mediated Conformational Activation. Science. 2014 Feb 6. PMID:24505130 doi:http://dx.doi.org/10.1126/science.1247997
- ↑ Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014 Feb 27;156(5):935-49. doi: 10.1016/j.cell.2014.02.001. Epub 2014 Feb, 13. PMID:24529477 doi:http://dx.doi.org/10.1016/j.cell.2014.02.001
- ↑ Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K, Thompson AJ, Nogales E, Doudna JA. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016 Jan 14. pii: aad8282. PMID:26841432 doi:http://dx.doi.org/10.1126/science.aad8282
- ↑ Wei Y, Terns RM, Terns MP. Cas9 function and host genome sampling in Type II-A CRISPR-Cas adaptation. Genes Dev. 2015 Feb 15;29(4):356-61. doi: 10.1101/gad.257550.114. PMID:25691466 doi:http://dx.doi.org/10.1101/gad.257550.114
- ↑ 17.0 17.1 17.2 Heler R, Samai P, Modell JW, Weiner C, Goldberg GW, Bikard D, Marraffini LA. Cas9 specifies functional viral targets during CRISPR-Cas adaptation. Nature. 2015 Mar 12;519(7542):199-202. doi: 10.1038/nature14245. Epub 2015 Feb, 18. PMID:25707807 doi:http://dx.doi.org/10.1038/nature14245
- ↑ 18.0 18.1 Nielsen AA, Voigt CA. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Mol Syst Biol. 2014 Nov 24;10:763. doi: 10.15252/msb.20145735. PMID:25422271
- ↑ 19.0 19.1 Didovyk A, Borek B, Hasty J, Tsimring L. Orthogonal Modular Gene Repression in Escherichia coli Using Engineered CRISPR/Cas9. ACS Synth Biol. 2016 Jan 15;5(1):81-8. doi: 10.1021/acssynbio.5b00147. Epub 2015 , Sep 30. PMID:26390083 doi:http://dx.doi.org/10.1021/acssynbio.5b00147
- ↑ 20.0 20.1 Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013 Jul 18;154(2):442-51. doi: 10.1016/j.cell.2013.06.044. Epub 2013 Jul, 11. PMID:23849981 doi:http://dx.doi.org/10.1016/j.cell.2013.06.044
- ↑ 21.0 21.1 Farzadfard F, Perli SD, Lu TK. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synth Biol. 2013 Oct 18;2(10):604-13. doi: 10.1021/sb400081r. Epub 2013 Sep, 11. PMID:23977949 doi:http://dx.doi.org/10.1021/sb400081r
- ↑ 22.0 22.1 Kiani S, Beal J, Ebrahimkhani MR, Huh J, Hall RN, Xie Z, Li Y, Weiss R. CRISPR transcriptional repression devices and layered circuits in mammalian cells. Nat Methods. 2014 Jul;11(7):723-6. doi: 10.1038/nmeth.2969. Epub 2014 May 5. PMID:24797424 doi:http://dx.doi.org/10.1038/nmeth.2969
- ↑ Nishimasu H, Cong L, Yan WX, Ran FA, Zetsche B, Li Y, Kurabayashi A, Ishitani R, Zhang F, Nureki O. Crystal Structure of Staphylococcus aureus Cas9. Cell. 2015 Aug 27;162(5):1113-26. doi: 10.1016/j.cell.2015.08.007. PMID:26317473 doi:http://dx.doi.org/10.1016/j.cell.2015.08.007