Urease
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
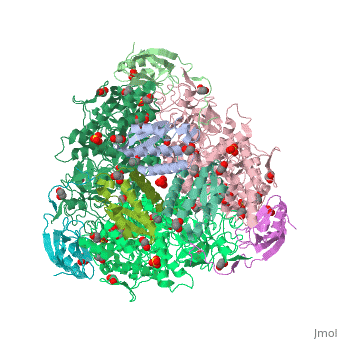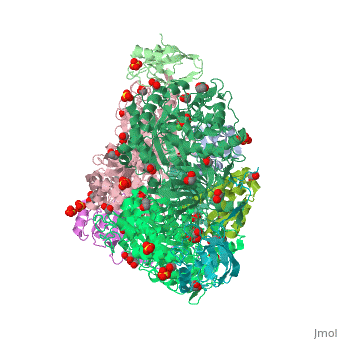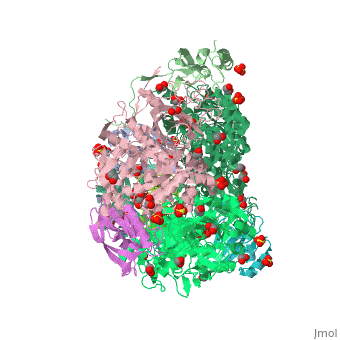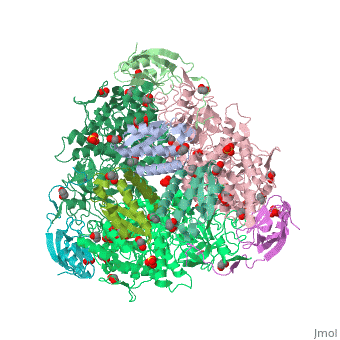
| - | <StructureSection load='4ac7' size=' | + | <StructureSection load='4ac7' size='350' side='right' scene='' caption='Urease α (pink), β (green), γ (grey) chains complex with citrate, sulfate and Ni+2 ions (green) (PDB code [[4ac7]])'> |
=Introduction= | =Introduction= | ||
'''Urease''' is a nickel-dependent metalloenzyme, is synthesized by plants, some bacteria, and fungi <ref name="urease">PMID: PMC2443974 </ref>. | '''Urease''' is a nickel-dependent metalloenzyme, is synthesized by plants, some bacteria, and fungi <ref name="urease">PMID: PMC2443974 </ref>. | ||
| Line 5: | Line 5: | ||
Ureases are among the few enzymes that require nickel for activity. It is known that binding of nickel to urease is very specific and tight and the removal of metal ions can be achieved only by harsh treatment with denaturants or acids,<ref name="nickel">Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480. </ref> which is not the case in most other metalloenzymes. In vivo incorporation of nickel in both bacterial and plant ureases requires a set of accessory proteins that appear to act as urease-specific chaperones <ref name="nickel2">Moncrief, M. C. & Hausinger, R. P. (1996). Nickel incorporation into urease. In Mechanisms of Metallo- center Assembly (Hausinger, R. P., Eichhorn, G. L. & Marzilli, L. G., eds), pp. 151–171, Elsevier Press, New York, NY. </ref>. | Ureases are among the few enzymes that require nickel for activity. It is known that binding of nickel to urease is very specific and tight and the removal of metal ions can be achieved only by harsh treatment with denaturants or acids,<ref name="nickel">Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480. </ref> which is not the case in most other metalloenzymes. In vivo incorporation of nickel in both bacterial and plant ureases requires a set of accessory proteins that appear to act as urease-specific chaperones <ref name="nickel2">Moncrief, M. C. & Hausinger, R. P. (1996). Nickel incorporation into urease. In Mechanisms of Metallo- center Assembly (Hausinger, R. P., Eichhorn, G. L. & Marzilli, L. G., eds), pp. 151–171, Elsevier Press, New York, NY. </ref>. | ||
One of the most common bacterial urease is the ''Helicobacter pylori'' since it has been implicated in peptic ulcers and stomach cancer <ref name="pylori">Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.</ref>. In plants, urease is widely distributed in leguminous seeds and is suggested to play an important role in seed germination<ref name="pylori">Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.</ref>. Plant ureases are also suggested to participate in seed chemical defenses <ref name="def">Polacco, J. C. & Holland, M. A. (1993). Roles of urease in plant cells. Int. Rev. Cytol. 145, 65–103.</ref>. | One of the most common bacterial urease is the ''Helicobacter pylori'' since it has been implicated in peptic ulcers and stomach cancer <ref name="pylori">Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.</ref>. In plants, urease is widely distributed in leguminous seeds and is suggested to play an important role in seed germination<ref name="pylori">Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.</ref>. Plant ureases are also suggested to participate in seed chemical defenses <ref name="def">Polacco, J. C. & Holland, M. A. (1993). Roles of urease in plant cells. Int. Rev. Cytol. 145, 65–103.</ref>. | ||
| + | |||
| + | See also [[Urease (Hebrew)]]. | ||
=Reaction= | =Reaction= | ||
| Line 15: | Line 17: | ||
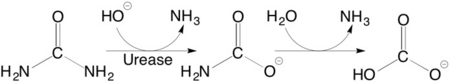
[[Urease]] ('''Urea Amidohydrolase''' [[EC]] [[Hydrolases|3.5.1.5]]) catalyzes the hydrolysis of urea to ammonia and carbon dioxide, thus allowing organisms to use exogenous and internally generated urea as a nitrogen source<ref name="urease">PMID: PMC2443974 </ref>. | [[Urease]] ('''Urea Amidohydrolase''' [[EC]] [[Hydrolases|3.5.1.5]]) catalyzes the hydrolysis of urea to ammonia and carbon dioxide, thus allowing organisms to use exogenous and internally generated urea as a nitrogen source<ref name="urease">PMID: PMC2443974 </ref>. | ||
| - | The multi-subunit enzyme usually has a 3:3 (alpha:beta) stoichiometry with a 2-fold symmetric structure (note that the image above gives the structure of the asymmetric unit, one-third of the true biological assembly). An exceptional urease is found in ''Helicobacter pylori'', which combines four of the regular six-subunit enzymes in an overall tetrahedral assembly of 24 subunits (α12β12). This supra-molecular assembly is thought to confer additional stability for the enzyme in this organism, which functions to produce ammonia in order to neutralise gastric acid. The presence of urease is used in | + | The multi-subunit enzyme usually has a 3:3 (alpha:beta) stoichiometry with a 2-fold symmetric structure (note that the image above gives the structure of the asymmetric unit, one-third of the true biological assembly). An exceptional urease is found in ''Helicobacter pylori'', which combines four of the regular six-subunit enzymes in an overall tetrahedral assembly of 24 subunits (α12β12). This supra-molecular assembly is thought to confer additional stability for the enzyme in this organism, which functions to produce ammonia in order to neutralise gastric acid. The presence of urease is used in t''Update February 2013''he diagnosis of Helicobacter species<ref name="characteristics">http://en.wikipedia.org/wiki/Urease </ref>. |
Molecular weight: 480 kDa or 545 kDa for Jack Bean Urease | Molecular weight: 480 kDa or 545 kDa for Jack Bean Urease | ||
| Line 66: | Line 68: | ||
crystal lattice, but <scene name='56/562376/Cv/5'>simply dimers</scene> arranged around the 6<sub>3</sub> axis, forming a large solvent channel. <scene name='56/562376/Cv/3'>The nickel-binding site in the center of SpUreE dimer is shown</scene>. The <scene name='56/562376/Cv/6'>second metal ion (site 2) was found in the N-terminal domain</scene>, linking <span style="color:salmon;background-color:black;font-weight:bold;">symmetry-related dimers (colored in salmon)</span>, and coordinated with a pseudo-tetrahedral geometry, interacting with <scene name='56/562376/Cv/7'>His9 and Asp12 as well as with the corresponding residues His9* and Asp12*</scene> from a symmetry-related dimer. In particular, the thermodynamic parameters of SpUreE for Ni(II) and Zn(II) binding have been determined using isothermal titration calorimetry. These experiments show that two Ni(II) ions bind to the protein dimer with positive cooperativity, with a high affinity and a low affinity site. Zn(II) binding to the protein, occurring in the same region and with similar affinity, causes metal-driven dimerization of the protein dimer. The crystal structure of the protein obtained in the presence of equimolar amounts of both metal ions indicates that the high affinity metal binding site preferentially binds Ni(II) over Zn(II). The ability of the protein to select Ni(II) over Zn(II) was confirmed by competition experiments in solution as well as by analysis of X-ray anomalous dispersion data. Overall, the thermodynamics and structural parameters that modulate the metal ion specificity of different binding sites on the protein surface have been established. | crystal lattice, but <scene name='56/562376/Cv/5'>simply dimers</scene> arranged around the 6<sub>3</sub> axis, forming a large solvent channel. <scene name='56/562376/Cv/3'>The nickel-binding site in the center of SpUreE dimer is shown</scene>. The <scene name='56/562376/Cv/6'>second metal ion (site 2) was found in the N-terminal domain</scene>, linking <span style="color:salmon;background-color:black;font-weight:bold;">symmetry-related dimers (colored in salmon)</span>, and coordinated with a pseudo-tetrahedral geometry, interacting with <scene name='56/562376/Cv/7'>His9 and Asp12 as well as with the corresponding residues His9* and Asp12*</scene> from a symmetry-related dimer. In particular, the thermodynamic parameters of SpUreE for Ni(II) and Zn(II) binding have been determined using isothermal titration calorimetry. These experiments show that two Ni(II) ions bind to the protein dimer with positive cooperativity, with a high affinity and a low affinity site. Zn(II) binding to the protein, occurring in the same region and with similar affinity, causes metal-driven dimerization of the protein dimer. The crystal structure of the protein obtained in the presence of equimolar amounts of both metal ions indicates that the high affinity metal binding site preferentially binds Ni(II) over Zn(II). The ability of the protein to select Ni(II) over Zn(II) was confirmed by competition experiments in solution as well as by analysis of X-ray anomalous dispersion data. Overall, the thermodynamics and structural parameters that modulate the metal ion specificity of different binding sites on the protein surface have been established. | ||
| - | + | = Evidence-based docking of the urease activation complex= | |
| - | + | Evidence-based docking of the <scene name='Journal:JBSD:6/Cv/11'>urease activation complex</scene> <ref>doi 10.1080/07391102.2012.713782</ref> | |
Ureases are enzymes that break down urea to carbon dioxide and ammonia, and they are one of the very few enzymes that have nickel in their active sites. Genetic and biochemical studies have shown that most of these enzymes require accessory proteins for the correct assembly of the nickel in their metallocenters. <scene name='Journal:JBSD:6/Cv/4'>UreA</scene> (<span style="color:lime;background-color:black;font-weight:bold;">green</span>), <scene name='Journal:JBSD:6/Cv/5'>UreB</scene> (<font color='red'><b>red</b></font>), and <scene name='Journal:JBSD:6/Cv/6'>UreC</scene> (<font color='darkmagenta'><b>darkmagenta</b></font>) form the <scene name='Journal:JBSD:6/Cv/7'>(UreABC)3 apoprotein</scene>. The trimeric representation | Ureases are enzymes that break down urea to carbon dioxide and ammonia, and they are one of the very few enzymes that have nickel in their active sites. Genetic and biochemical studies have shown that most of these enzymes require accessory proteins for the correct assembly of the nickel in their metallocenters. <scene name='Journal:JBSD:6/Cv/4'>UreA</scene> (<span style="color:lime;background-color:black;font-weight:bold;">green</span>), <scene name='Journal:JBSD:6/Cv/5'>UreB</scene> (<font color='red'><b>red</b></font>), and <scene name='Journal:JBSD:6/Cv/6'>UreC</scene> (<font color='darkmagenta'><b>darkmagenta</b></font>) form the <scene name='Journal:JBSD:6/Cv/7'>(UreABC)3 apoprotein</scene>. The trimeric representation | ||
considers UreABC as a functional unit. Studies of ''Klebsiella aerogenes'' urease activation pathway revealed that three accessory proteins – <span style="color:yellow;background-color:black;font-weight:bold;">UreD (yellow)</span>, <span style="color:cyan;background-color:black;font-weight:bold;">UreF (cyan)</span>, <font color='magenta'><b>UreG (magenta)</b></font> – are essential for the production of a functional urease. These proteins sequentially bind to form the <scene name='Journal:JBSD:6/Cv/8'>(UreABC-UreD)3</scene>, <scene name='Journal:JBSD:6/Cv/9'>(UreABC-UreDF)3</scene>, and <scene name='Journal:JBSD:6/Cv/10'>(UreABC-UreDFG)3</scene> activation complexes. <scene name='Journal:JBSD:6/Cv/12'>Click here to see this structure</scene> is rotated by 90º. In this work we submitted structural models of such proteins to macromolecular docking calculations with ''K. aerogenes'' urease, which lead to a putative structure for the urease activation complex. | considers UreABC as a functional unit. Studies of ''Klebsiella aerogenes'' urease activation pathway revealed that three accessory proteins – <span style="color:yellow;background-color:black;font-weight:bold;">UreD (yellow)</span>, <span style="color:cyan;background-color:black;font-weight:bold;">UreF (cyan)</span>, <font color='magenta'><b>UreG (magenta)</b></font> – are essential for the production of a functional urease. These proteins sequentially bind to form the <scene name='Journal:JBSD:6/Cv/8'>(UreABC-UreD)3</scene>, <scene name='Journal:JBSD:6/Cv/9'>(UreABC-UreDF)3</scene>, and <scene name='Journal:JBSD:6/Cv/10'>(UreABC-UreDFG)3</scene> activation complexes. <scene name='Journal:JBSD:6/Cv/12'>Click here to see this structure</scene> is rotated by 90º. In this work we submitted structural models of such proteins to macromolecular docking calculations with ''K. aerogenes'' urease, which lead to a putative structure for the urease activation complex. | ||
| Line 84: | Line 86: | ||
{{Clear}} | {{Clear}} | ||
The present study consistently supports an interaction of fluoride with the nickel centres in the urease active site in which <scene name='59/596313/Cv/17'>one fluoride competitively binds</scene> (<span style="color:salmon;background-color:black;font-weight:bold;">colored in salmon</span>) to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while <scene name='59/596313/Cv/18'>another fluoride uncompetitively substitutes</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">colored in cyan</span>) the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea. | The present study consistently supports an interaction of fluoride with the nickel centres in the urease active site in which <scene name='59/596313/Cv/17'>one fluoride competitively binds</scene> (<span style="color:salmon;background-color:black;font-weight:bold;">colored in salmon</span>) to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while <scene name='59/596313/Cv/18'>another fluoride uncompetitively substitutes</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">colored in cyan</span>) the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea. | ||
| + | =3D structures of urease= | ||
| + | [[Urease 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
__NOTOC__ | __NOTOC__ | ||
| - | =3D structures of urease= | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *Urease | ||
| - | |||
| - | **[[2kau]], [[1kra]], [[1fwj]], [[1ejx]], [[1ejw]], [[4ep8]] – KaUA α+β+γ chains – ''Klebsiella aerogenes''<br /> | ||
| - | **[[1ef2]] - KaUA α+β+γ chains Mn substituted<br /> | ||
| - | **[[1krb]], [[1krc]], [[1fwa]], [[1fwb]], [[1fwc]], [[1fwd]], [[1fwf ]], [[1fwg]], [[1fwh]], [[1fwi]] – KaUA α (mutant) +β (mutant) +γ (mutant) chains<br /> | ||
| - | **[[1a5k]], [[1a5l]], [[1a5m]], [[1ejr]], [[1ejs]], [[1ejt]], [[1eju]], [[1ejv]] - KaUA α+β+γ (mutant) chains<br /> | ||
| - | **[[2ubp]] - BpUA α+β+γ chains – ''Bacillus pasteurii''<br /> | ||
| - | **[[1e9z]] - HpUA α+β chains – ''Helicobacter pylori''<br /> | ||
| - | **[[3qga]], [[3qgk]] - UA β/γ chains Fe containing – ''Helicobacter mustelae''<br /> | ||
| - | **[[2fvh]] - UA γ chain – ''Mycobacterium tuberculosis''<br /> | ||
| - | **[[3la4]] – UA – horse bean<br /> | ||
| - | **[[4epb]], [[4epd]], [[4epe]] - UA α+β+γ chains – ''Enterobacter aerogenes''<br /> | ||
| - | **[[4ac7]] - UA α+β+γ chains – ''Sporosarcina pasteurii''<br /> | ||
| - | **[[4fur]] - UA γ2 chain – ''Enterobacter melitensis''<br /> | ||
| - | **[[4g7e]] - UA – pigeon pea<br /> | ||
| - | **[[4gy7]] - jbUA – jack bean<br /> | ||
| - | |||
| - | *Urease binary complex | ||
| - | **[[1a5n]], [[1a5o]] - KaUA α+β+γ (mutant) chains + formate<br /> | ||
| - | **[[1fwe]] – KaUA α (mutant) +β (mutant) +γ (mutant) chains + acetohydroxamic acid<br /> | ||
| - | **[[1ubp]] - BpUA α+β+γ chains + mercaptoethanol <br /> | ||
| - | **[[3ubp]] - BpUA α+β+γ chains + diamidophosphate<br /> | ||
| - | **[[4ubp]] - BpUA α+β+γ chains + acetohydroxamic acid<br /> | ||
| - | **[[1ie7]] - BpUA α+β+γ chains + phosphate<br /> | ||
| - | **[[1s3t]] - BpUA α+β+γ chains + borate<br /> | ||
| - | **[[1e9y]] - HpUA α+β chains + acetohydroxamic acid<br /> | ||
| - | **[[4goa]] - jbUA + F <br /> | ||
| - | **[[4h9m]] - jbUA + acetohydroxamic acid<br /> | ||
| - | }} | ||
=Additional Resources= | =Additional Resources= | ||
For additional information on Urinary Tract Infection, See: [[1tr7]] <br /> | For additional information on Urinary Tract Infection, See: [[1tr7]] <br /> | ||
Current revision
| |||||||||||
Additional Resources
For additional information on Urinary Tract Infection, See: 1tr7
For additional information on Helicobacter Pylori, See: 1e9z
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 PMID: PMC2443974
- ↑ http://www.jbc.org/content/277/35/e23.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&searchid=1130442887043_7599&stored_search=&FIRSTINDEX=60&tocsectionid=Classics&sortspec=PUBDATE_SORTDATE+desc
- ↑ Andrews, R. K., Blakeley, R. L. & Zerner, B. (1984). Urea and urease. Adv. Inorg. Biochem. 6, 245–283.
- ↑ Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480.
- ↑ Moncrief, M. C. & Hausinger, R. P. (1996). Nickel incorporation into urease. In Mechanisms of Metallo- center Assembly (Hausinger, R. P., Eichhorn, G. L. & Marzilli, L. G., eds), pp. 151–171, Elsevier Press, New York, NY.
- ↑ 6.0 6.1 Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.
- ↑ Polacco, J. C. & Holland, M. A. (1993). Roles of urease in plant cells. Int. Rev. Cytol. 145, 65–103.
- ↑ 8.0 8.1 http://en.wikipedia.org/wiki/Urease
- ↑ 9.0 9.1 9.2 Mobley, H. L. T., Island, M. D. & Hausinger, R. P. (1995). Molecular biology of microbial ureases. Microbiol. Rev. 59, 451–480.
- ↑ http://www.cell.com/structure/abstract/S0969-2126(99)80026-4#.
- ↑ Cicmanec JF, Helmers SL, Evans AT. Office practice survey of urease positive bacterial pathogens causing urinary tract infections. Urology. 1980 Sep;16(3):274-6. PMID:6999699
- ↑ Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480.
- ↑ Becker-Ritt, A. B., Martinelli, A. H. S., Mitidieri, S., Feder, V., Wassermann, G. E., Santi, L. et al. (2007). Antifungal activity of plant and bacterial ureases. Toxicon, 50, 971–983.
- ↑ 14.0 14.1 Follmer, C., Real-Guerra, R., Wassermann, G. E., Olivera-Severo, D. & Carlini, C. R. (2004). Jackbean, soybean and Bacillus pasteurii ureases—biological effects unrelated to ureolytic activity. Eur. J. Biochem. 271, 1357–1363.
- ↑ Karplus, P. A., Pearson, M. A. & Hausinger, R. P. (1997). 70 years of crystalline urease: what have we learnt? Acc. Chem. Res. 30, 330–337.
- ↑ Benini, S., Rypneiwski, W. R., Wilson, K. S., Meletti, S., Ciurli, S. & Mangani, S. (1999). A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure, 7, 205–216.
- ↑ 17.0 17.1 http://tonga.usip.edu/jsnow/chem348/recitation8.pdf
- ↑ http://emedicine.medscape.com/article/1174503-overview
- ↑ http://www.nucdf.org/ucd_treatment.htm
- ↑ Benini S, Kosikowska P, Cianci M, Mazzei L, Vara AG, Berlicki L, Ciurli S. The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design. J Biol Inorg Chem. 2013 Mar;18(3):391-9. doi: 10.1007/s00775-013-0983-7. Epub, 2013 Feb 15. PMID:23412551 doi:10.1007/s00775-013-0983-7
- ↑ Kcx - Lysine NZ-carboxylic acid
- ↑ Zambelli B, Banaszak K, Merloni A, Kiliszek A, Rypniewski W, Ciurli S. Selectivity of Ni(II) and Zn(II) binding to Sporosarcina pasteurii UreE, a metallochaperone in the urease assembly: a calorimetric and crystallographic study. J Biol Inorg Chem. 2013 Dec;18(8):1005-17. doi: 10.1007/s00775-013-1049-6. Epub, 2013 Oct 15. PMID:24126709 doi:http://dx.doi.org/10.1007/s00775-013-1049-6
- ↑ Ligabue-Braun R, Real-Guerra R, Carlini CR, Verli H. Evidence-based docking of the urease activation complex. J Biomol Struct Dyn. 2012 Sep 10. PMID:22962938 doi:10.1080/07391102.2012.713782
- ↑ Benini S, Cianci M, Mazzei L, Ciurli S. Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics. J Biol Inorg Chem. 2014 Aug 12. PMID:25113581 doi:http://dx.doi.org/10.1007/s00775-014-1182-x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Andrea Graydon, Alexander Berchansky, David Canner, OCA