Johnson's Monday Lab Sandbox for Insulin Receptor
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
==Structure== | ==Structure== | ||
The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a heterotetramer which is constructed from two homodimers. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The extracellular domain is divided into alpha and beta subunits. The alpha subunit is characterized by two leucine-rich regions and one cysteine rich region. The beta subunit contains three fibronectin type III domains. The alpha and beta subunits of the extracellular domains fold over one another and form a "V" shape when the insulin receptor is unactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a "T" shape. | The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a heterotetramer which is constructed from two homodimers. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The extracellular domain is divided into alpha and beta subunits. The alpha subunit is characterized by two leucine-rich regions and one cysteine rich region. The beta subunit contains three fibronectin type III domains. The alpha and beta subunits of the extracellular domains fold over one another and form a "V" shape when the insulin receptor is unactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a "T" shape. | ||
| - | [[Image: | + | [[Image:Insulin Receptor T.jpg|thumb|right|250px|Figure 1:Insulin receptor in the "T" shape active conformation with four insulins bound]] |
An additional component to the ectodomain is the ''alpha'' chain C-terminal helix <ref name= "Uchikawa" />. The ''alpha''-CT is a single alpha helix and it plays an important role in insulin binding and stabilization of the "T" shape activated conformation. The ''alpha''-CT interacts with a leucine rich region of the alpha subunit and a fibronectin type III region of the beta subunit to form an insulin binding site <ref name="Uchikawa" />. | An additional component to the ectodomain is the ''alpha'' chain C-terminal helix <ref name= "Uchikawa" />. The ''alpha''-CT is a single alpha helix and it plays an important role in insulin binding and stabilization of the "T" shape activated conformation. The ''alpha''-CT interacts with a leucine rich region of the alpha subunit and a fibronectin type III region of the beta subunit to form an insulin binding site <ref name="Uchikawa" />. | ||
| Line 26: | Line 26: | ||
===Conformational Changes=== | ===Conformational Changes=== | ||
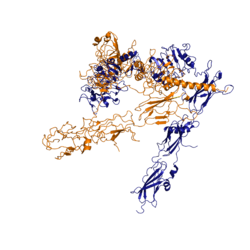
| - | [[Image:image 6.png|thumb|left|250px|Figure | + | [[Image:image 6.png|thumb|left|250px|Figure 2:Conformational change of insulin receptor protomer upon insulin binding. Blue is inactivated. Orange is activated.]] |
STABILIZING INTERACTIONS. HOW DOES THE SHAPE CHANGE? WHEN IS CHANGE DRASTIC? NOT MUCH IS KNOWN ABOUT HOW THIS HAPPENS. | STABILIZING INTERACTIONS. HOW DOES THE SHAPE CHANGE? WHEN IS CHANGE DRASTIC? NOT MUCH IS KNOWN ABOUT HOW THIS HAPPENS. | ||
| + | |||
==Type II Diabetes== | ==Type II Diabetes== | ||
WHAT IS T2D? HOW DOES IT RELATE TO THE INSULIN RECEPTOR? WHAT DOES A NON T2D SYSTEM LOOK LIKE? HOW DOES IT COMPARE TO T1D? WHY IS THIS IMPORTANT? | WHAT IS T2D? HOW DOES IT RELATE TO THE INSULIN RECEPTOR? WHAT DOES A NON T2D SYSTEM LOOK LIKE? HOW DOES IT COMPARE TO T1D? WHY IS THIS IMPORTANT? | ||
Revision as of 00:05, 24 March 2020
Insulin Receptor
| |||||||||||