Johnson's Monday Lab Sandbox for Insulin Receptor
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
The insulin receptor resides within the [http://en.wikipedia.org/wiki/Cell_membrane plasma membrane] of insulin target cells of different organs, such as the liver, and tissues including skeletal muscle and adipose. Activation of the insulin receptor is dependent upon insulin binding. Once activated, the receptor serves as the gateway for the regulation of various cellular processes. These processes include but are not limited to glucose transport, glycogen storage, [http://en.wikipedia.org/wiki/Autophagy autophagy], [http://en.wikipedia.org/wiki/Apoptosis apoptosis], and gene expression. Additionally, the insulin receptor has been associated with the development of diseases such as Alzheimer's, Type II Diabetes, and cancer <ref name="Scapin" />. Characterization of the structure of the insulin receptor, as well as understanding of the molecular mechanisms which initiate a [http://en.wikipedia.org/wiki/Conformational_change conformational change], are important for understanding the role that the insulin receptor plays within a cell and in the development of the disease. | The insulin receptor resides within the [http://en.wikipedia.org/wiki/Cell_membrane plasma membrane] of insulin target cells of different organs, such as the liver, and tissues including skeletal muscle and adipose. Activation of the insulin receptor is dependent upon insulin binding. Once activated, the receptor serves as the gateway for the regulation of various cellular processes. These processes include but are not limited to glucose transport, glycogen storage, [http://en.wikipedia.org/wiki/Autophagy autophagy], [http://en.wikipedia.org/wiki/Apoptosis apoptosis], and gene expression. Additionally, the insulin receptor has been associated with the development of diseases such as Alzheimer's, Type II Diabetes, and cancer <ref name="Scapin" />. Characterization of the structure of the insulin receptor, as well as understanding of the molecular mechanisms which initiate a [http://en.wikipedia.org/wiki/Conformational_change conformational change], are important for understanding the role that the insulin receptor plays within a cell and in the development of the disease. | ||
==Insulin== | ==Insulin== | ||
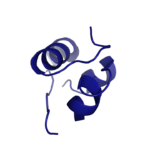
| - | [[Image:Insulin.png|thumb|right|150px|Figure 1: Insulin molecule]] Insulin is a [http://en.wikipedia.org/wiki/Hormone hormone] that is synthesized and secreted from the pancreas in response to high concentrations of glucose in the blood. Once it is secreted, it will move through the bloodstream and attach to an insulin receptor. Once multiple insulins are bound to the receptor, it is activated and as mentioned previously, the regulation of various cellular processes is initiated. | + | [[Image:Insulin.png|thumb|right|150px|Figure 1: Insulin molecule]] Insulin is a [http://en.wikipedia.org/wiki/Hormone hormone] that is synthesized and secreted from the [https://en.wikipedia.org/wiki/Pancreatic_islets islets of Langerhans] of the pancreas in response to high concentrations of glucose in the blood. Once it is secreted, it will move through the bloodstream and attach to an insulin receptor. Once multiple insulins are bound to the receptor, it is activated and as mentioned previously, the regulation of various cellular processes is initiated. |
==Structure== | ==Structure== | ||
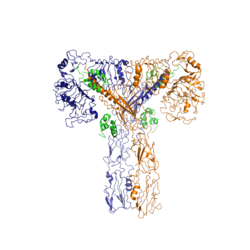
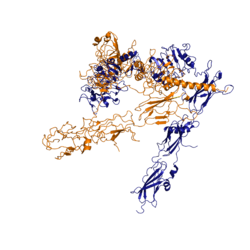
The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a heterotetramer that is constructed from two homodimers. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The extracellular domain is divided into alpha and beta [http://en.wikipedia.org/wiki/Protein_subunit subunit]. The alpha subunit is characterized by two leucine-rich regions and one cysteine-rich region. The beta subunit contains three fibronectin type III domains. The alpha and beta subunits of the extracellular domains fold over one another and form a "V" shape when the insulin receptor is inactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a "T" shape. | The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a heterotetramer that is constructed from two homodimers. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The extracellular domain is divided into alpha and beta [http://en.wikipedia.org/wiki/Protein_subunit subunit]. The alpha subunit is characterized by two leucine-rich regions and one cysteine-rich region. The beta subunit contains three fibronectin type III domains. The alpha and beta subunits of the extracellular domains fold over one another and form a "V" shape when the insulin receptor is inactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a "T" shape. | ||
Revision as of 20:19, 26 March 2020
Insulin Receptor
| |||||||||||