Johnson's Monday Lab Sandbox for Insulin Receptor
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
===Insulin Binding=== | ===Insulin Binding=== | ||
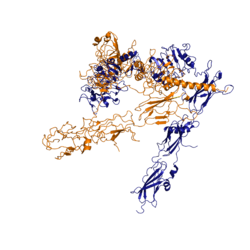
| - | The insulin receptor unit has four separate sites for the insulin binding. There are two pairs of two identical binding sites referred to as <scene name='83/839263/Insulin_molecules_at_site_1/1'>sites 1 and 1'</scene> and <scene name='83/839263/Insulin_molecules_at_site_2/1'>sites 2 and 2'</scene>. The insulin molecules bind to these sites mostly through [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic interactions], with some of the most crucial residues at sites 1 and 1' being between <scene name='83/839263/Residues_of_site_1_binding/8'>Cys A7, Cys B7, and His B5 of insulin and Pro495, Phe497, and Arg498</scene> of the insulin receptor FnIII-1 domain <ref name="Uchikawa" />. Despite some of the residues included being charged they can still interact hydrophobically in this binding site. For example, due to arginine carrying its positive charge at the end of the side chain, <scene name='83/839263/Arginine_bending/1'> the side chain is bent</scene> to allow the hydrophobic part of the side chain to interact with the other hydrophobic residues. At sites 2 and 2', the major residues contributing to these hydrophobic interactions are the <scene name='83/839263/Site_2_residues_hydrophobic/4'>Leu 486, Leu 552, and Pro537 of the insulin receptor and Leu A13, Try A14, Leu A16, Leu B6, Ala B14, Leu B17 and Val B18 of the insulin molecule</scene><ref name="Uchikawa" />. While the majority of the binding interactions appear similar, sites 1 and 1' have a higher binding affinity than sites 2 and 2' due to site 1 having a larger surface area (706 Å<sup>2</sup>) exposed for insulin to bind to compared to site 2 (394 Å<sup>2</sup>)<ref name="Uchikawa" />. The binding interactions of the insulin molecules in sites 1 and 1' are facilitated by hydrophobic residues of an <scene name='83/839263/Insulin_bound_to_site_1/4'>alpha-helix</scene> of the insulin receptor. The insulin molecules in sites 2 and 2' primarily interact with the residues that comprise some of the <scene name='83/839263/Insulin_in_site_2_with_beta_sh/ | + | The insulin receptor unit has four separate sites for the insulin binding. There are two pairs of two identical binding sites referred to as <scene name='83/839263/Insulin_molecules_at_site_1/1'>sites 1 and 1'</scene> and <scene name='83/839263/Insulin_molecules_at_site_2/1'>sites 2 and 2'</scene>. The insulin molecules bind to these sites mostly through [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic interactions], with some of the most crucial residues at sites 1 and 1' being between <scene name='83/839263/Residues_of_site_1_binding/8'>Cys A7, Cys B7, and His B5 of insulin and Pro495, Phe497, and Arg498</scene> of the insulin receptor FnIII-1 domain <ref name="Uchikawa" />. Despite some of the residues included being charged they can still interact hydrophobically in this binding site. For example, due to arginine carrying its positive charge at the end of the side chain, <scene name='83/839263/Arginine_bending/1'> the side chain is bent</scene> to allow the hydrophobic part of the side chain to interact with the other hydrophobic residues. At sites 2 and 2', the major residues contributing to these hydrophobic interactions are the <scene name='83/839263/Site_2_residues_hydrophobic/4'>Leu 486, Leu 552, and Pro537 of the insulin receptor and Leu A13, Try A14, Leu A16, Leu B6, Ala B14, Leu B17 and Val B18 of the insulin molecule</scene><ref name="Uchikawa" />. While the majority of the binding interactions appear similar, sites 1 and 1' have a higher binding affinity than sites 2 and 2' due to site 1 having a larger surface area (706 Å<sup>2</sup>) exposed for insulin to bind to compared to site 2 (394 Å<sup>2</sup>)<ref name="Uchikawa" />. The binding interactions of the insulin molecules in sites 1 and 1' are facilitated by hydrophobic residues of an <scene name='83/839263/Insulin_bound_to_site_1/4'>alpha-helix</scene> of the insulin receptor. The insulin molecules in sites 2 and 2' primarily interact with the residues that comprise some of the<scene name='83/839263/Insulin_in_site_2_with_beta_sh/7'>beta-sheets</scene> of the insulin receptor. The secondary structures themselves are not what directly causes the differences in binding affinities, but the surface area that the insulin molecule can interact with. |
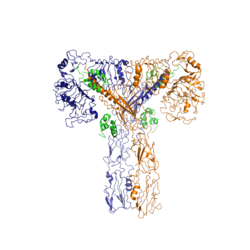
At least three insulin molecules have to bind to the insulin receptor to induce the active <scene name='83/839263/T-shape/4'>"T" shape</scene> conformation <ref name="Uchikawa" />. The difference between the fully bound state with four insulins and the three-insulin-bound state is minimal compared to the difference between two and three insulins bound <ref name="Uchikawa" />. However, binding only two insulin molecules is insufficient to move the receptor to the active "T" shape <ref name="Uchikawa" />. | At least three insulin molecules have to bind to the insulin receptor to induce the active <scene name='83/839263/T-shape/4'>"T" shape</scene> conformation <ref name="Uchikawa" />. The difference between the fully bound state with four insulins and the three-insulin-bound state is minimal compared to the difference between two and three insulins bound <ref name="Uchikawa" />. However, binding only two insulin molecules is insufficient to move the receptor to the active "T" shape <ref name="Uchikawa" />. | ||
Revision as of 23:01, 16 April 2020
Insulin Receptor
| |||||||||||