Johnson's Monday Lab Sandbox for Insulin Receptor
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
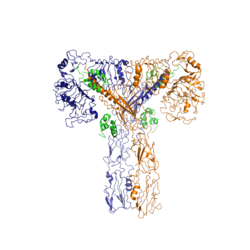
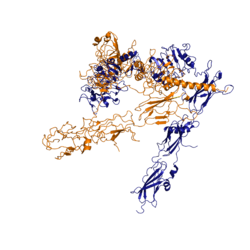
The insulin receptor resides within the [http://en.wikipedia.org/wiki/Cell_membrane plasma membrane] of insulin targeted cells of various organs and tissues, including the liver, skeletal muscle, and adipose. The insulin receptor is activated by multiple insulin molecules binding to various sites on the receptor. Once activated, the receptor serves as the gateway for the regulation of various cellular processes including glucose transport, glycogen storage, [http://en.wikipedia.org/wiki/Autophagy autophagy], [http://en.wikipedia.org/wiki/Apoptosis apoptosis], and gene expression. Additionally, problems with the insulin receptor are associated with the development of diseases such as Alzheimer's, type II diabetes, and cancer <ref name="Scapin" />. Recent structures of the insulin receptor have illustrated the large scale [http://en.wikipedia.org/wiki/Conformational_change conformational changes], intiated by insulin binding. | The insulin receptor resides within the [http://en.wikipedia.org/wiki/Cell_membrane plasma membrane] of insulin targeted cells of various organs and tissues, including the liver, skeletal muscle, and adipose. The insulin receptor is activated by multiple insulin molecules binding to various sites on the receptor. Once activated, the receptor serves as the gateway for the regulation of various cellular processes including glucose transport, glycogen storage, [http://en.wikipedia.org/wiki/Autophagy autophagy], [http://en.wikipedia.org/wiki/Apoptosis apoptosis], and gene expression. Additionally, problems with the insulin receptor are associated with the development of diseases such as Alzheimer's, type II diabetes, and cancer <ref name="Scapin" />. Recent structures of the insulin receptor have illustrated the large scale [http://en.wikipedia.org/wiki/Conformational_change conformational changes], intiated by insulin binding. | ||
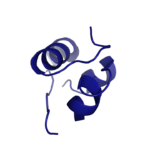
==Insulin== | ==Insulin== | ||
| - | [[Image:Insulin.png|thumb|right|150px|Figure 1: Insulin molecule]] The <scene name='83/839263/Insulin_molecule/3'>insulin molecule</scene> is a [http://en.wikipedia.org/wiki/Hormone hormone] made of two seperate amino acid chains that is synthesized and secreted from the [http://en.wikipedia.org/wiki/Pancreatic_islets islets of Langerhans] of the pancreas in response to high concentrations of glucose in the blood. Once it is secreted, insulin moves through the bloodstream and binds to unactivated insulin receptors residing in the plasma membrane. The receptor is fully activated after multiple insulin molecules are bound, | + | [[Image:Insulin.png|thumb|right|150px|Figure 1: Insulin molecule]] The <scene name='83/839263/Insulin_molecule/3'>insulin molecule</scene> is a [http://en.wikipedia.org/wiki/Hormone hormone] made of two seperate amino acid chains that is synthesized and secreted from the [http://en.wikipedia.org/wiki/Pancreatic_islets islets of Langerhans] of the pancreas in response to high concentrations of glucose in the blood. Once it is secreted, insulin moves through the bloodstream and binds to unactivated insulin receptors residing in the plasma membrane. The receptor is fully activated after multiple insulin molecules are bound, through a complex process. |
==Structure== | ==Structure== | ||
The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a [http://en.wikipedia.org/wiki/Heterotetramer heterotetramer] that is constructed from two [http://en.wiktionary.org/wiki/homodimer homodimers]. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The insulin is divided into <scene name='83/839263/Alpha_and_beta_subunit/3'>alpha and beta</scene> [http://en.wikipedia.org/wiki/Protein_subunit subunits]. The alpha subunit is characterized by two leucine-rich regions and one cysteine-rich region. The beta subunit contains three fibronectin type III domains along with the transmembrane domain and intracellular tyrosine kinase domain that could not be shown in one continous PDB structure. The alpha and beta subunits of the extracellular domains fold over one another and form a <scene name='83/839263/V_shape/3'>"V" shape</scene> when the insulin receptor is inactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a <scene name='83/839263/T-shape/4'>"T" shape</scene>. | The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a [http://en.wikipedia.org/wiki/Heterotetramer heterotetramer] that is constructed from two [http://en.wiktionary.org/wiki/homodimer homodimers]. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The insulin is divided into <scene name='83/839263/Alpha_and_beta_subunit/3'>alpha and beta</scene> [http://en.wikipedia.org/wiki/Protein_subunit subunits]. The alpha subunit is characterized by two leucine-rich regions and one cysteine-rich region. The beta subunit contains three fibronectin type III domains along with the transmembrane domain and intracellular tyrosine kinase domain that could not be shown in one continous PDB structure. The alpha and beta subunits of the extracellular domains fold over one another and form a <scene name='83/839263/V_shape/3'>"V" shape</scene> when the insulin receptor is inactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a <scene name='83/839263/T-shape/4'>"T" shape</scene>. | ||
| Line 13: | Line 13: | ||
An additional component to the [http://en.wikipedia.org/wiki/Ectodomain ectodomain] is the <scene name='83/839263/Alpha-ct/2'> ''alpha'' chain C-terminal helix</scene>, which is also referred to as the "''alpha''-CT" <ref name= "Uchikawa" />. Each of the dimers has an "alpha"-CT. The ''alpha''-CT is a single alpha-helix and it plays an important role in insulin binding and stabilization of the "T" shape activated conformation. The ''alpha''-CT interacts with a leucine-rich region of the alpha subunit and a fibronectin type III region of the beta subunit to form the insulin binding sites known as <scene name='83/839263/Insulin_molecules_at_site_1/1'>site 1 and site 1'</scene> <ref name="Uchikawa" />. | An additional component to the [http://en.wikipedia.org/wiki/Ectodomain ectodomain] is the <scene name='83/839263/Alpha-ct/2'> ''alpha'' chain C-terminal helix</scene>, which is also referred to as the "''alpha''-CT" <ref name= "Uchikawa" />. Each of the dimers has an "alpha"-CT. The ''alpha''-CT is a single alpha-helix and it plays an important role in insulin binding and stabilization of the "T" shape activated conformation. The ''alpha''-CT interacts with a leucine-rich region of the alpha subunit and a fibronectin type III region of the beta subunit to form the insulin binding sites known as <scene name='83/839263/Insulin_molecules_at_site_1/1'>site 1 and site 1'</scene> <ref name="Uchikawa" />. | ||
| - | The structure of the extracellular domain is stabilized through [ | + | The structure of the extracellular domain is stabilized through multiple [https://en.wikipedia.org/wiki/Disulfide disulfide bonds]. The alpha subunits are linked through two disulfide bonds, with the main one being between <scene name='83/839263/Cys_holding_alphas_together/4'>Cys524</scene> of one alpha subunit and <scene name='83/839263/Cys_holding_alphas_together/4'>Cys524</scene> of the other alpha subunit <ref name="Schäffer" />. <scene name='83/839263/Cys_683_holding_alphas_togethe/3'>Cys683</scene> of both alpha subunits hold the two together with a disulfide bond <ref name="Sparrow" />. The alpha subunit is held to the beta subunit by a disulfide bond between the <scene name='83/839263/Alpha_beta_link_by_disulfide/5'>Cys647 of the alpha subunit and Cys872 of the beta subunit</scene><ref name="Sparrow" />. |
The insulin receptor extends intracellularly from the beta subunits of the ectodomain by way of a [http://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] helix. Intracellularly, the insulin receptor contains two tyrosine kinase domains. | The insulin receptor extends intracellularly from the beta subunits of the ectodomain by way of a [http://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] helix. Intracellularly, the insulin receptor contains two tyrosine kinase domains. | ||
Revision as of 18:07, 19 April 2020
Insulin Receptor
| |||||||||||