Johnson's Monday Lab Sandbox for Insulin Receptor
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 8: | Line 8: | ||
==Structure== | ==Structure== | ||
The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a [http://en.wikipedia.org/wiki/Heterotetramer heterotetramer] that is constructed from two [http://en.wiktionary.org/wiki/homodimer homodimers]. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The insulin receptor is divided into <scene name='83/839263/Alpha_and_beta_subunit/3'>alpha and beta</scene> [http://en.wikipedia.org/wiki/Protein_subunit subunits]. The alpha subunit is characterized by two leucine-rich regions and one cysteine-rich region. The beta subunit contains three fibronectin type III domains along with the transmembrane domain and intracellular tyrosine kinase domain that could not be shown in one continous PDB structure. The alpha and beta subunits of the extracellular domains fold over one another and form a <scene name='83/839263/V_shape/3'>"V" shape</scene> when the insulin receptor is inactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a <scene name='83/839263/T-shape/4'>"T" shape</scene>. | The insulin receptor is a [http://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase]. It is a [http://en.wikipedia.org/wiki/Heterotetramer heterotetramer] that is constructed from two [http://en.wiktionary.org/wiki/homodimer homodimers]. Each homodimer maintains an extracellular domain, transmembrane helix, and an intracellular domain. The insulin receptor is divided into <scene name='83/839263/Alpha_and_beta_subunit/3'>alpha and beta</scene> [http://en.wikipedia.org/wiki/Protein_subunit subunits]. The alpha subunit is characterized by two leucine-rich regions and one cysteine-rich region. The beta subunit contains three fibronectin type III domains along with the transmembrane domain and intracellular tyrosine kinase domain that could not be shown in one continous PDB structure. The alpha and beta subunits of the extracellular domains fold over one another and form a <scene name='83/839263/V_shape/3'>"V" shape</scene> when the insulin receptor is inactivated. Upon activation, the extracellular domain undergoes a conformational change and forms a <scene name='83/839263/T-shape/4'>"T" shape</scene>. | ||
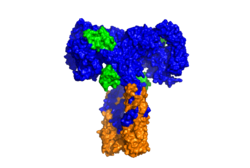
| - | [[Image:SurfaceIR.png|thumb|right|250px|Figure 2: Surface | + | [[Image:SurfaceIR.png|thumb|right|250px|Figure 2: Surface representation of the insulin receptor in the active "T" shape conformation with four insulins bound (green). PDB: 6sof]] |
An additional component to the [http://en.wikipedia.org/wiki/Ectodomain ectodomain] is the <scene name='83/839263/Alpha-ct/2'> ''alpha'' chain C-terminal helix</scene>, which is also referred to as the "''alpha''-CT" <ref name= "Uchikawa" />. Each of the dimers has an "alpha"-CT. The ''alpha''-CT is a single alpha-helix and it plays an important role in insulin binding and stabilization of the "T" shape activated conformation. The ''alpha''-CT interacts with a leucine-rich region of the alpha subunit and a fibronectin type III region of the beta subunit to form the insulin binding sites known as <scene name='83/839263/Insulin_molecules_at_site_1/1'>site 1 and site 1'</scene> <ref name="Uchikawa" />. | An additional component to the [http://en.wikipedia.org/wiki/Ectodomain ectodomain] is the <scene name='83/839263/Alpha-ct/2'> ''alpha'' chain C-terminal helix</scene>, which is also referred to as the "''alpha''-CT" <ref name= "Uchikawa" />. Each of the dimers has an "alpha"-CT. The ''alpha''-CT is a single alpha-helix and it plays an important role in insulin binding and stabilization of the "T" shape activated conformation. The ''alpha''-CT interacts with a leucine-rich region of the alpha subunit and a fibronectin type III region of the beta subunit to form the insulin binding sites known as <scene name='83/839263/Insulin_molecules_at_site_1/1'>site 1 and site 1'</scene> <ref name="Uchikawa" />. | ||
| Line 24: | Line 24: | ||
As the fibronectin type III domains of the beta subunit swing inward, the alpha subunits also undergo a conformational change upon insulin binding. As insulin binds to site 1, the leucine-rich region of one protomer interacts with the ''alpha''-CT and the FNIII-1 domains of the other protomer to form a binding site. These interactions are referred to as the <scene name='83/839263/Tripartite_interface/2'>tripartite interface</scene> <ref name="Uchikawa" />. In order for the tripartite interface to form, the alpha subunits of each protomer must undergo a "folding" motion. | As the fibronectin type III domains of the beta subunit swing inward, the alpha subunits also undergo a conformational change upon insulin binding. As insulin binds to site 1, the leucine-rich region of one protomer interacts with the ''alpha''-CT and the FNIII-1 domains of the other protomer to form a binding site. These interactions are referred to as the <scene name='83/839263/Tripartite_interface/2'>tripartite interface</scene> <ref name="Uchikawa" />. In order for the tripartite interface to form, the alpha subunits of each protomer must undergo a "folding" motion. | ||
| - | The proper conformational change of the ectodomain of the insulin receptor is crucial for transmitting the signal into the cell. The movements extracellularly cause the two receptor tyrosine kinase domains intracellularly to become close enough to each other to [ | + | The proper conformational change of the ectodomain of the insulin receptor is crucial for transmitting the signal into the cell. The movements extracellularly cause the two receptor tyrosine kinase domains intracellularly to become close enough to each other to [http://en.wikipedia.org/wiki/Autophosphorylation autophosphorylate] <ref name="Boucher" />. This autophosphorylation leads enzymes to become activated in the cell that carries out processes related to insulin signaling such as metabolism and growth <ref name="Boucher" />. |
While there is an explanation for which conformational changes of the insulin receptor take place, there is no full explanation for the exact mechanism by which the conformational changes are executed in the receptor <ref name="Uchikawa" />. It is known where the various domains move, but not the specifics for how this is achieved on the atomic level due to the complexity of analyzing moving structures. | While there is an explanation for which conformational changes of the insulin receptor take place, there is no full explanation for the exact mechanism by which the conformational changes are executed in the receptor <ref name="Uchikawa" />. It is known where the various domains move, but not the specifics for how this is achieved on the atomic level due to the complexity of analyzing moving structures. | ||
==Type II Diabetes== | ==Type II Diabetes== | ||
| - | [http://en.wikipedia.org/wiki/Type_2_diabetes Type II diabetes] (T2D) is a chronic condition that affects 10 percent of the world's population <ref name="Boucher" />. T2D is characterized by insulin resistance and leads to high concentrations of glucose in the bloodstream. A type II diabetic produces insulin, but when the insulin molecule binds to the insulin receptor, the signal is not properly transmitted intracellularly <ref name="Boucher" />. Insulin resistance in routine type II diabetics is not associated with mutations of the insulin receptor gene, but instead, the signal being disrupted later in the pathway <ref name="Boucher" />. Mutations of the receptor gene are associated with more severe cases of insulin resistance, as seen in leprechaunism | + | [http://en.wikipedia.org/wiki/Type_2_diabetes Type II diabetes] (T2D) is a chronic condition that affects 10 percent of the world's population <ref name="Boucher" />. T2D is characterized by insulin resistance and leads to high concentrations of glucose in the bloodstream. A type II diabetic produces insulin, but when the insulin molecule binds to the insulin receptor, the signal is not properly transmitted intracellularly <ref name="Boucher" />. Insulin resistance in routine type II diabetics is not associated with mutations of the insulin receptor gene, but instead, the signal being disrupted later in the pathway <ref name="Boucher" />. Mutations of the receptor gene are associated with more severe cases of insulin resistance, as seen in [http://en.wikipedia.org/wiki/Donohue_syndrome leprechaunism]. Additionally, mutations of the insulin receptor can be fatal, as it is crucial for many cellular processes including gene expression, glucose homeostasis, and apoptosis <ref name="Boucher" />. The basis for insulin resistance in typical type II diabetics is complex and cannot yet be explained by one particular factor <ref name="Franks" /> <ref name="Boucher" />. |
There are a multitude of hypotheses which discuss the reasons for the establishment of type II diabetes <ref name="Boucher" /> <ref name="Franks" />. While the specifics of the development of T2D are beyond the scope of this article, it is important to note that the molecular causes for insulin resistance and T2D have been primarily attributed to the inhibition of key proteins involved in the insulin signaling and glucose transport pathway <ref name="Boucher" />. Alterations to the phosphorylation cascade of insulin signaling can be the result of changes within the cellular environment including [http://en.wikipedia.org/wiki/Lipotoxicity lipotoxicity], inflammation, [http://en.wikipedia.org/wiki/Hyperglycemia hyperglycemia], and the presence of [http://en.wikipedia.org/wiki/Reactive_oxygen_species reactive oxygen species] (ROS) <ref name="Boucher" />. On a macroscopic level, a variety of factors influence the cellular environment, and thus the risk for T2D. These factors include gestational environment, [http://en.wikipedia.org/wiki/Human_microbiome microbiome], genetics, diet, and energy expenditure <ref name="Franks" />. Recent studies, which have evaluated the relationships between genetics and environmental factors in the progress of T2D, have shown that T2D is not uniform among the population and the biochemistry behind the development of risk factors varies for each patient <ref name="Franks" />. | There are a multitude of hypotheses which discuss the reasons for the establishment of type II diabetes <ref name="Boucher" /> <ref name="Franks" />. While the specifics of the development of T2D are beyond the scope of this article, it is important to note that the molecular causes for insulin resistance and T2D have been primarily attributed to the inhibition of key proteins involved in the insulin signaling and glucose transport pathway <ref name="Boucher" />. Alterations to the phosphorylation cascade of insulin signaling can be the result of changes within the cellular environment including [http://en.wikipedia.org/wiki/Lipotoxicity lipotoxicity], inflammation, [http://en.wikipedia.org/wiki/Hyperglycemia hyperglycemia], and the presence of [http://en.wikipedia.org/wiki/Reactive_oxygen_species reactive oxygen species] (ROS) <ref name="Boucher" />. On a macroscopic level, a variety of factors influence the cellular environment, and thus the risk for T2D. These factors include gestational environment, [http://en.wikipedia.org/wiki/Human_microbiome microbiome], genetics, diet, and energy expenditure <ref name="Franks" />. Recent studies, which have evaluated the relationships between genetics and environmental factors in the progress of T2D, have shown that T2D is not uniform among the population and the biochemistry behind the development of risk factors varies for each patient <ref name="Franks" />. | ||
Current revision
Insulin Receptor
| |||||||||||