|
Introduction
The RNA dependent RNA polymerase (RdRp) of SARS-CoV-2 (also known as nsp12), which is responsible for a major outbreak of the disease called Coronavirus Disease 2019 (COVID-19), declared pandemic by WHO in 11 march 2020 [1] is an enzyme that catalyzes the synthesis of RNA of the virus SARS-CoV-2.[2] This virus belongs to the betacoronavirus genus[3] and has a single stranded positive-sense RNA genome. Therefore, RNA dependent RNA polymerase plays a central role in the virus replication and livecycle. It’s also has been considered a good target for antiviral drugs.[2]
Function
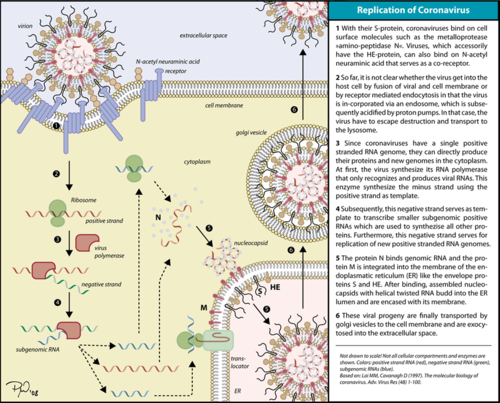 Corona virus replication cycle. The RdRp is responsible for replication and transcription of the virus genome alongside with other non-structural proteins (NSPs). After the firsts stages of infection, follow by the translation and assembly of the replicase complex, this enzyme helps the synthesis of genomic and sub-genomic RNA. Sub-genomic RNAs are used as mRNA for structural and accessory proteins.[4] The RdRp synthesizes negative-sense RNA and uses it as template for positive-sense strands for RNA replication for packaging into virus particles, and sub-genomic RNA transcription for translation.[5] Moreover, it has been reported that RdRp forms a complex with two other non-structural proteins NSP7 and NSP8, which act as cofactors and confer processivity to its RNA-synthesizing activity.[6][7]
Structure
Overview
The RdRp bonded to NSP7 and NSP8 consists of an approximately 160 kDa protein complex.[8] The RNA dependent RNA polymerase of SARS-CoV-2 contains 942 amino acid residues while NSP7 has 198 and NSP8 83 residues. (To view the primary and secondary structure of SARS-CoV-2 RdRp and its cofactors visit https://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=6m71). The structure of RdRp protein of COVID-19 virus contains : a “right hand” (residues S367-F920), a (residues D60-R249), which has a nidovirus RdRp-associated nucleotidyltransferase domain (NiRAN) architecture, an (residues A250-R365) that connects the RdRp domain and NiRAN domain, and a newly identified β-hairpin domain at its N terminus (residues D29-K50).[2]
RdRp domain
The RdRp domain has a conserved architectures and comprises : (residues L366-A581 and K621-G679), a (residues T582-P620 and T680-Q815), and a (residues H816-E920).[2] Its active site consists of the polymerase motifs A, B, C, D, E, F, and G, with important (759-SDD-761) been located in Motif C, in the palm subdomain, in the turn of the loop of .[2] As in other viral RNA polymerases, [9] the template-directed RNA synthesis is mediated by the RdRp domain motifs.[2]
NiRAN and β-hairpin domain
The complete SARS-CoV-2 RdRp protein structure obtained by cryo-EM[2][10] allowed to resolve the N-terminal portion of the NiRAN domain as well to identify a N-terminal β-hairpin domain, what was in part unresolved for SARS-CoV RdRp protein.[8] It was found that this β-hairpin is inserted in the groove clamped by the NiRAN domain and the palm subdomain and it forms close contacts that help to stabilize the overall structure.[2]
An attractive drug target
As has been shown, SARS-CoV-2 RdRp protein is crucial for viral replication and so, it has been an important drug target in the fight against COVID-19.[2][11] In fact, growing attention has been given to RdRp as target of a class of antiviral drugs known as nucleotide analogs, including Remdesivir.[10][12] Remdesivir is an adenosine monophosphate analog and it is converted into its active drug form (its triphosphate form) within the cells.[13] It is positioned at the center of the catalytic active site and, as for other nucleotide analogs, Remdesivir was shown to inhibits the RdRp activity through non-obligate RNA chain termination, which requires the conversion of its monophosphate form to the triphosphate one.[10] In a recent study[10] (10), it was described some differences between the apo and the complex (with Remdesivir and a template RNA) structures, showing some small conformational changes between them. (The three dimensional structure of the nsp12-nsp7-nsp8 complex bounded to the template-primer RNA and triphosphate form of Remdesivir can be seen in https://www.rcsb.org/3d-view/7BV2).
References
- ↑ WHO. COVID-19 situation reports [Internet]. [cited 2020 May 15]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 PMID: 32277040.
- ↑ Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270-273. doi: 10.1038/s41586-020-2012-7. Epub 2020 Feb, 3. PMID:32015507 doi:http://dx.doi.org/10.1038/s41586-020-2012-7
- ↑ Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Helena Jane Maier et al. (eds) Coronaviruses: Methods and Protocols, Methods in Molecular Biology. 2015; 1282:1-23. Springer, New York. DOI 10.1007/978-1-4939-2438-7_1
- ↑ Lai, Michael M. C., and David Cavanagh. 1997. ‘The Molecular Biology of Coronaviruses’. In Advances in Virus Research, edited by Karl Maramorosch, Frederick A. Murphy, and Aaron J. Shatkin, 48:1–100. Academic Press. https://doi.org/10.1016/S0065-3527(08)60286-9.
- ↑ Zhai Y, Sun F, Li X, Pang H, Xu X, Bartlam M, Rao Z. Insights into SARS-CoV transcription and replication from the structure of the nsp7-nsp8 hexadecamer. Nat Struct Mol Biol. 2005 Nov;12(11):980-6. PMID:16228002 doi:10.1038/nsmb999
- ↑ Subissi L, Posthuma CC, Collet A, Zevenhoven-Dobbe JC, Gorbalenya AE, Decroly E, Snijder EJ, Canard B, Imbert I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):E3900-9. doi:, 10.1073/pnas.1323705111. Epub 2014 Sep 2. PMID:25197083 doi:http://dx.doi.org/10.1073/pnas.1323705111
- ↑ 8.0 8.1 Kirchdoerfer, Robert N., and Andrew B. Ward. 2019. ‘Structure of the SARS-CoV Nsp12 Polymerase Bound to Nsp7 and Nsp8 Co-Factors’. Nature Communications 10 (1): 2342 https://doi.org/10.1038/s41467-019-10280-3
- ↑ Gong P, Peersen OB. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 2010 Dec 10. PMID:21148772 doi:10.1073/pnas.1007626107
- ↑ 10.0 10.1 10.2 10.3 Yin W, Mao C, Luan X, Shen DD, Shen Q, Su H, Wang X, Zhou F, Zhao W, Gao M, Chang S, Xie YC, Tian G, Jiang HW, Tao SC, Shen J, Jiang Y, Jiang H, Xu Y, Zhang S, Zhang Y, Xu HE. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020 May 1. pii: science.abc1560. doi: 10.1126/science.abc1560. PMID:32358203 doi:http://dx.doi.org/10.1126/science.abc1560
- ↑ Pokhrel, Rudramani, Prem Chapagain, and Jessica Siltberg-Liberles. 2020. ‘Potential RNA-Dependent RNA Polymerase Inhibitors as Prospective Therapeutics against SARS-CoV-2’. Journal of Medical Microbiology. https://doi.org/10.1099/jmm.0.001203.
- ↑ Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Mar;30(3):269-271. doi: 10.1038/s41422-020-0282-0. Epub 2020 Feb, 4. PMID:32020029 doi:http://dx.doi.org/10.1038/s41422-020-0282-0
- ↑ Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, Mackman RL. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem. 2017 Mar 9;60(5):1648-1661. doi: 10.1021/acs.jmedchem.6b01594. Epub, 2017 Feb 14. PMID:28124907 doi:http://dx.doi.org/10.1021/acs.jmedchem.6b01594
|

