Methylenetetrahydrofolate reductase
From Proteopedia
m (User:Shaylie Albright/MTHFR moved to MTHFR) |
m (MTHFR moved to Methylenetetrahydrofolate reductase) |
Revision as of 15:13, 11 April 2022
MTHFR is an enzyme.
Contents |
Function
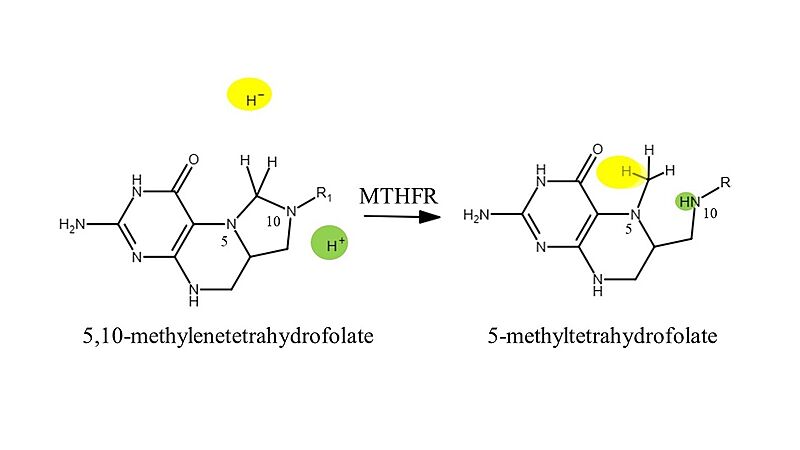
Methylenetetrahydrofolate reductase (MTHFR) is a regulatory agent of one carbon folate metabolism. The enzyme catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate to be recycled back into the folate cycle, and for aiding folate uptake in the body. This reduction reaction requires the cofactor molecule flavin adenine dinucleotide (FAD) and the second substrate nicotinamide adenine dinucleotide phosphate (NADPH) as the electron donor in the reaction. MTHFR has a unique folding structure. Its N-terminal is abundant in serine and acts as a phosphorylation site, its situated in close proximity to it's C-terminal S-adenosyl methionine (SAM) binding site. A linker joins the catalytic domain (N-terminal) to the regulatory domain (C-terminal) for interaction and increases the sensitivity to SAM binding and feedback properties.
Disease
In addition to the folate cycle, MTHFR is also a major component of the homeostasis of homocysteine in the blood stream. When this homeostasis is disrupted, mutations are created that result in hyperhomocysteinemia with homocystinuria, or mild hyperhomocysteinemia. Hyperhomocysteinemia is an excess of the amino acid circulating in the body, and is a direct correlation of cardiovascular disease, Alzheimer's disease, depression, and neural tube defects within the fetus. Furthermore, homocystinuria is clinically described as the body's inability to adequately process homocysteine and the amino acid methionine. This dysfunction can be clinically presented with skeletal, vision, and blood clotting abnormalities coupled with learning disorders.
Relevance
| |||||||||||
References
Proteopedia Page Contributors and Editors (what is this?)
Shaylie Albright, Karsten Theis, Michal Harel, Michael O'Shaughnessy, Kia Yang, Anna Postnikova