Journal:Acta Cryst D:S205979832100677X
From Proteopedia
(Difference between revisions)

| (3 intermediate revisions not shown.) | |||
| Line 9: | Line 9: | ||
The dimer formation of ''Wc''AG: | The dimer formation of ''Wc''AG: | ||
*<scene name='88/886503/Cv/28'>First view, each subunit is colored in different colors</scene>. | *<scene name='88/886503/Cv/28'>First view, each subunit is colored in different colors</scene>. | ||
| - | *<scene name='88/886503/Cv/ | + | *<scene name='88/886503/Cv/29'>Second view, each domain of subunit is colored in different colors</scene>. Blue represents Domain A, whereas Domain B, C, and N are shown in yellow, red, and green, respectively. Lighter colors represent different subunit of dimer. |
| - | + | ||
| - | + | ||
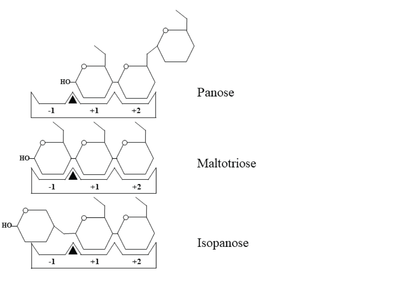
| - | ''Wc''AG formed a <scene name='88/886503/Cv/ | + | ''Wc''AG formed a <scene name='88/886503/Cv/30'>homodimer, of which the N-terminal domain of one monomer orientated in proximity to the catalytic domain of another</scene>, creating the substrate-binding groove. The residues near the dimer interface are shown in cyan and lavender spacefill representation, the maltotriose is shown in ball and stick representation and colored in yellow, the catalytic residues are colored magenta. |
Ligand binding sites: | Ligand binding sites: | ||
| Line 34: | Line 32: | ||
<scene name='88/886503/Cv2/13'>Superimposition of WcAG bound with acarbose and TvAII complexed with maltohexaose</scene> (PDB ID: [[2d2o]]). The acabose is displayed in cyan, while yellow represents maltohexose. The residues E374Q, D345, and D440 are catalytic residues. The asterisk (*) indicates the residues from another subunit. | <scene name='88/886503/Cv2/13'>Superimposition of WcAG bound with acarbose and TvAII complexed with maltohexaose</scene> (PDB ID: [[2d2o]]). The acabose is displayed in cyan, while yellow represents maltohexose. The residues E374Q, D345, and D440 are catalytic residues. The asterisk (*) indicates the residues from another subunit. | ||
| + | |||
| + | '''PDB references:''' α-glucosidase from ''Weisella cibaria'' BBK-1, wild type, [[7d9b]]; E374Q mutant, complex with maltose, [[7d9c]]; E374Q mutant, complex with maltotriose, [[7dcg]]; E374Q mutant, complex with acarbose, [[7dch]]; D345N mutant, complex with maltose, [[7ehh]]; D345N mutant, covalent maltosyl-α-glucosidase intermediate, [[7ehi]]. | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.