Acetyl-CoA carboxylase
From Proteopedia
(Difference between revisions)
(New page: <!-- Please use the "3D" button above this box to insert a Jmol applet (molecule) on this page. Or use the four-green-boxes-button to insert scrollable text adjacent to a Jmol applet. Che...) |
|||
| (42 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
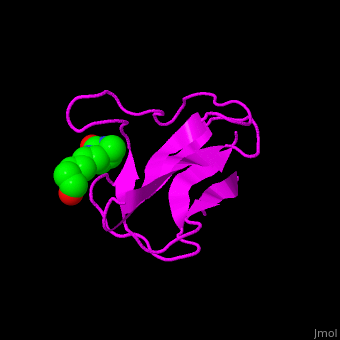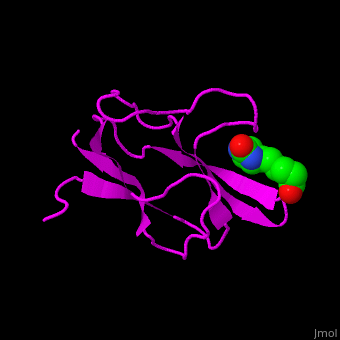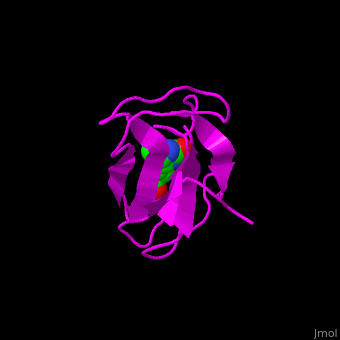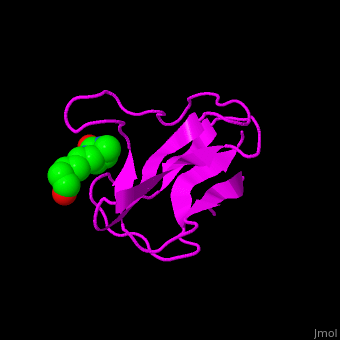
| - | < | + | <StructureSection load='' size='350' side='right' scene='49/492046/Cv/1' caption='E. coli Acetyl-CoA carboxylase biotinyl domain complex with biotin (PDB code [[1bdo]]) '> |
| - | + | __TOC__ | |
| - | + | == Function == | |
| - | + | '''Acetyl-CoA carboxylase''' (ACC) catalyzes the irreversible carboxylation of <scene name='43/430893/Cv/2'>acetyl-CoA</scene> to <scene name='49/492046/Cv/7'>malonyl-CoA</scene>. Malonyl-CoA is a building block in in the biosynthesis of fatty acids. ACC is biotin- and ATP-dependent enzyme. In mammals, 2 forms of ACC exist. ACC1 and ACC2 differ in their tissue distribution and function. See also [[3-Hydroxypropionate bicycle]]. | |
| - | + | == Structural highlights == | |
| - | '''Acetyl-CoA carboxylase''' (ACC) catalyzes the irreversible carboxylation of acetyl-CoA to malonyl-CoA. | + | ACC is a multi-subunit enzyme in prokaryotes and plants. Each subunit catalyzes different reaction. These are – '''biotin carboxylase''' (BC) which carboxylates the biotin prosthetic group, '''biotin carboxyl carrier protein''' (BCCP) which is linked covalently to biotin and '''carboxyltransferase''' (CT) which transfers the carboxyl group from biotin to acetyl-CoA. In eukaryotes these functions are performed by a single polypeptide chain. The biotin moiety is located in the beta turn connecting the N and C halves of BCCP. <scene name='49/492046/Cv/6'>E.coli biotin carboxylase complex with biotin</scene> ([[1bdo]]) is shown. <ref>PMID:8747466</ref> Water molecules shown as red spheres. |
| - | == | + | == Disease == |
| - | + | Bacterial ACC serves as a potential drug target for novel antibiotics. | |
| - | + | ==3D structures of acetyl-CoA carboxylase== | |
| - | + | [[Acetyl-CoA carboxylase 3D structures]] | |
| - | [[ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[ | + | == References == |
| + | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Athappilly FK, Hendrickson WA. Structure of the biotinyl domain of acetyl-coenzyme A carboxylase determined by MAD phasing. Structure. 1995 Dec 15;3(12):1407-19. PMID:8747466