|
|
| (2 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| | | | |
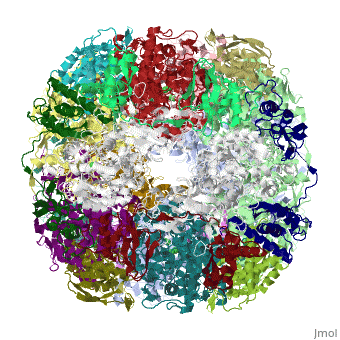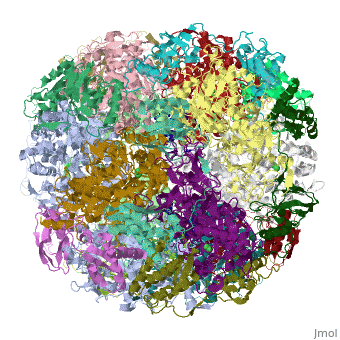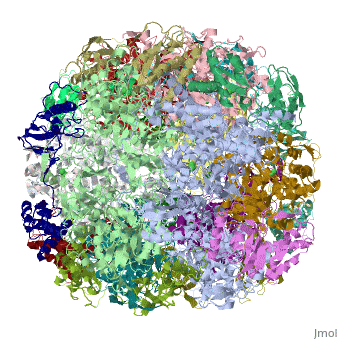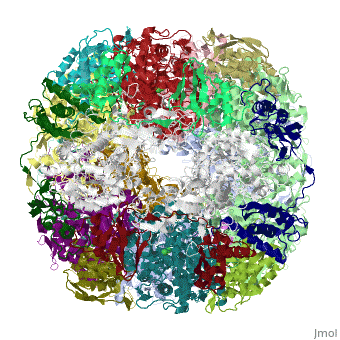
| | ==Crystal structure of Helicobacter pylori urease== | | ==Crystal structure of Helicobacter pylori urease== |
| - | <StructureSection load='1e9z' size='340' side='right' caption='[[1e9z]], [[Resolution|resolution]] 3.00Å' scene=''> | + | <StructureSection load='1e9z' size='340' side='right'caption='[[1e9z]], [[Resolution|resolution]] 3.00Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[1e9z]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Atcc_43504 Atcc 43504]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1E9Z OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1E9Z FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[1e9z]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Helicobacter_pylori Helicobacter pylori]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1E9Z OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1E9Z FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=NI:NICKEL+(II)+ION'>NI</scene></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3Å</td></tr> |
| - | <tr id='NonStdRes'><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=KCX:LYSINE+NZ-CARBOXYLIC+ACID'>KCX</scene></td></tr> | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=KCX:LYSINE+NZ-CARBOXYLIC+ACID'>KCX</scene>, <scene name='pdbligand=NI:NICKEL+(II)+ION'>NI</scene></td></tr> |
| - | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1e9y|1e9y]]</td></tr>
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1e9z FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e9z OCA], [https://pdbe.org/1e9z PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1e9z RCSB], [https://www.ebi.ac.uk/pdbsum/1e9z PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1e9z ProSAT]</span></td></tr> |
| - | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Urease Urease], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.5.1.5 3.5.1.5] </span></td></tr>
| + | |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1e9z FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e9z OCA], [http://pdbe.org/1e9z PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1e9z RCSB], [http://www.ebi.ac.uk/pdbsum/1e9z PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=1e9z ProSAT]</span></td></tr> | + | |
| | </table> | | </table> |
| | == Function == | | == Function == |
| - | [[http://www.uniprot.org/uniprot/URE23_HELPY URE23_HELPY]] Ammonia produced by ureolysis increases the gastric pH thereby providing an environment permissive for colonization of the stomach.<ref>PMID:8039935</ref> [[http://www.uniprot.org/uniprot/URE1_HELPY URE1_HELPY]] Ammonia produced by ureolysis increases the gastric pH thereby providing an environment permissive for colonization of the stomach.<ref>PMID:8039935</ref> | + | [https://www.uniprot.org/uniprot/URE23_HELPY URE23_HELPY] Ammonia produced by ureolysis increases the gastric pH thereby providing an environment permissive for colonization of the stomach.<ref>PMID:8039935</ref> |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 34: |
Line 32: |
| | ==See Also== | | ==See Also== |
| | *[[Urease|Urease]] | | *[[Urease|Urease]] |
| - | *[[Urease (Hebrew)|Urease (Hebrew)]] | + | *[[Urease 3D structures|Urease 3D structures]] |
| | == References == | | == References == |
| | <references/> | | <references/> |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| - | [[Category: Atcc 43504]] | + | [[Category: Helicobacter pylori]] |
| - | [[Category: Urease]] | + | [[Category: Large Structures]] |
| - | [[Category: Ha, N C]] | + | [[Category: Ha N-C]] |
| - | [[Category: Oh, B H]] | + | [[Category: Oh B-H]] |
| - | [[Category: Oh, S T]] | + | [[Category: Oh S-T]] |
| - | [[Category: Hydrolase]]
| + | |
| Structural highlights
Function
URE23_HELPY Ammonia produced by ureolysis increases the gastric pH thereby providing an environment permissive for colonization of the stomach.[1]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Helicobacter pylori, an etiologic agent in a variety of gastroduodenal diseases, produces a large amount of urease, which is believed to neutralize gastric acid by producing ammonia for the survival of the bacteria. Up to 30% of the enzyme associates with the surface of intact cells upon lysis of neighboring bacteria. The role of the enzyme at the extracellular location has been a subject of controversy because the purified enzyme is irreversibly inactivated below pH 5. We have determined the crystal structure of H. pylori urease, which has a 1.1 MDa spherical assembly of 12 catalytic units with an outer diameter of approximately 160 A. Under physiologically relevant conditions, the activity of the enzyme remains unaffected down to pH 3. Activity assays under different conditions indicated that the cluster of the 12 active sites on the supramolecular assembly may be critical for the survival of the enzyme at low pH. The structure provides a novel example of a molecular assembly adapted for acid resistance that, together with the low Km value of the enzyme, is likely to enable the organism to inhabit the hostile niche.
Supramolecular assembly and acid resistance of Helicobacter pylori urease.,Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH Nat Struct Biol. 2001 Jun;8(6):505-9. PMID:11373617[2]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Tsuda M, Karita M, Morshed MG, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994 Aug;62(8):3586-9. PMID:8039935
- ↑ Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Biol. 2001 Jun;8(6):505-9. PMID:11373617 doi:10.1038/88563
|