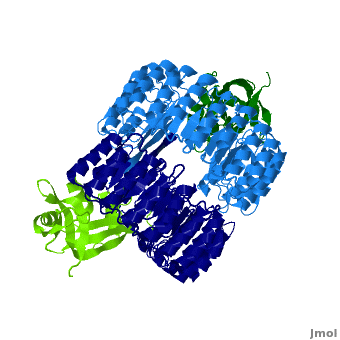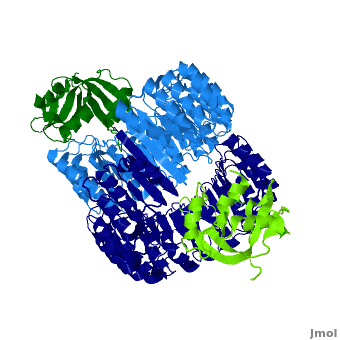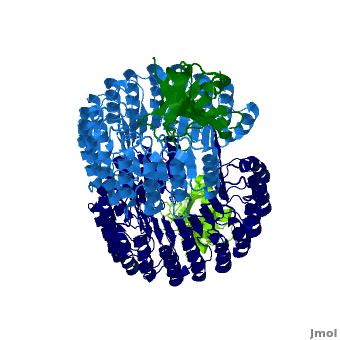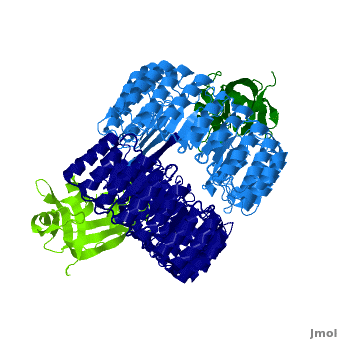
Ribonuclease inhibitor
From Proteopedia
(Difference between revisions)
| (30 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | '''Ribonuclease inhibitors (RI)''' are a family of large (~450 residues, ~49 kDa), acidic (pI ~4.7), proteins that | + | <structuresection load='' size='450' caption='hRI (blue and dark blue) dimer complexed with RNase 1 (light green and dark green), [[1z7x]]' scene='Ribonuclease_inhibitor/1z7x/3' > |
| + | ==Function== | ||
| + | '''Ribonuclease inhibitors (RI)''' or '''Ribonuclease/angiogenin inhibitor''' are a family of large (~450 residues, ~49 kDa), acidic (pI ~4.7), proteins that bind to and inhibit [http://en.wikipedia.org/wiki/Ribonuclease ribonucleases]. Human RI(hRI) is a major cellular protein, comprising ~0.1% of all cellular protein by weight. <ref>PMID: 11582809</ref> | ||
'''Ribonucleases (RNase)''' are enzymes that degrade RNA and are often [http://en.wikipedia.org/wiki/Cytotoxicity cytotoxic] which gives them chemotherapeutic properties. However, when bound to an RI they are no longer functional. Understanding the mechanism through which RI identifies and binds to RNases will allow scientists to design/modify RNases to evade hRI. In fact, one drug, Onconase (ONC), a ribonuclease from the Northern Leopard Frog ([http://en.wikipedia.org/wiki/Northern_Leopard_Frog Rana pipiens]), is now in Phase III clinical trials as a cancer chemotherapeutic agent <ref>PMID:20173221</ref>. | '''Ribonucleases (RNase)''' are enzymes that degrade RNA and are often [http://en.wikipedia.org/wiki/Cytotoxicity cytotoxic] which gives them chemotherapeutic properties. However, when bound to an RI they are no longer functional. Understanding the mechanism through which RI identifies and binds to RNases will allow scientists to design/modify RNases to evade hRI. In fact, one drug, Onconase (ONC), a ribonuclease from the Northern Leopard Frog ([http://en.wikipedia.org/wiki/Northern_Leopard_Frog Rana pipiens]), is now in Phase III clinical trials as a cancer chemotherapeutic agent <ref>PMID:20173221</ref>. | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Structural Motifs== | ==Structural Motifs== | ||
| - | RI features 15 leucine-rich-repeats (LRR) alternating 28 and 29 residues. <scene name='Ribonuclease_inhibitor/Motiffs/8'>Alpha helices</scene> make up the outer circumference with <scene name='Ribonuclease_inhibitor/Motiffs/9'>parallel beta sheets</scene> on the inner circumference. There are no disulfide bonds in the <scene name='Ribonuclease_inhibitor/Motiffs/10'>horseshoe shaped structure.</scene> While RI undergoes a conformation change upon binding of a substrate, there is no hinge. The lack of long-range stabilization allows for structural flexibility especially between the two ends of the molecule. | + | RI features 15 leucine-rich-repeats (LRR) alternating 28 and 29 residues. <scene name='Ribonuclease_inhibitor/Motiffs/8'>Alpha helices</scene> make up the outer circumference with <scene name='Ribonuclease_inhibitor/Motiffs/9'>parallel beta sheets</scene> on the inner circumference. There are no disulfide bonds in the <scene name='Ribonuclease_inhibitor/Motiffs/10'>horseshoe shaped structure.</scene> While RI undergoes a conformation change upon binding of a substrate, there is no hinge. The lack of long-range stabilization allows for structural flexibility especially between the two ends of the molecule<ref> PMID:9000628 </ref>. |
| - | <br><scene name='Ribonuclease_inhibitor/Motiffs/5'>Spacefilling</scene><br> | + | <br><scene name='Ribonuclease_inhibitor/Motiffs/5'>Spacefilling</scene><br> |
| - | + | ||
| - | + | ||
==Interactions between hRI and RNase 1== | ==Interactions between hRI and RNase 1== | ||
| Line 15: | Line 13: | ||
<br> hRI + RNase 1 ←→ hRI·RNase 1 <br> | <br> hRI + RNase 1 ←→ hRI·RNase 1 <br> | ||
| - | Arg39 and Arg91 of RNase 1 are proposed to be “electrostatic targeting residues” a term used by Johnson et. al to define residues that push the formation of protein complexes<ref>PMID:17350650</ref> As shown, <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> and <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> form multiple hydrogen bonds to hRI, keeping the RNase in place, allowing the formation of salt bridges that further lock hRI and RNase together. The targeting residues hold the complex together while a total of nineteen intermolecular hydrogen bonds form<ref>PMID:17350650</ref>. | + | Arg39 and Arg91 of RNase 1 are proposed to be “electrostatic targeting residues” a term used by Johnson et. al to define residues that push the formation of protein complexes<ref>PMID:17350650</ref> As shown, <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> and <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> form multiple hydrogen bonds to hRI, keeping the RNase in place, allowing the formation of salt bridges that further lock hRI and RNase together. The targeting residues hold the complex together while a total of nineteen intermolecular hydrogen bonds form. This includes a multitude of salt bridges which are especially strong. |
| + | <br>'''Hydrogen Bonds:'''<br> | ||
| + | RNase 1:::::::hRI<br> | ||
| + | Arg4:::::::Trp438<br> | ||
| + | Arg4:::::::Trp438<br> | ||
| + | Lys7:::::::Ser460<br> | ||
| + | Gln11:::::::Ser460<br> | ||
| + | Arg31:::::::Gln10<br> | ||
| + | Arg31:::::::Asp36<br> | ||
| + | Arg32:::::::Asp36<br> | ||
| + | Arg32:::::::Asp36<br> | ||
| + | Arg39:::::::Glu401<br> | ||
| + | Arg39:::::::Glu401<br> | ||
| + | Arg39:::::::Glu401<br> | ||
| + | Lys41:::::::Asp435<br> | ||
| + | Asn67:::::::Tyr437<br> | ||
| + | Asn71:::::::Tyr437<br> | ||
| + | Asn88:::::::Glu264<br> | ||
| + | Gly89:::::::Trp261<br> | ||
| + | Arg91:::::::Glu287<br> | ||
| + | Arg91:::::::Glu287<br> | ||
| + | Glu111:::::::Tyr437.<ref>PMID:17350650</ref> | ||
| + | <br> | ||
| + | [http://www.youtube.com/watch?v=cZhAwiG81_Q RI + RNase 1] | ||
==Mechanism of Inhibition== | ==Mechanism of Inhibition== | ||
| - | One remarkable property of RI is the ability to recognize | + | One remarkable property of RI is the ability to recognize, bind, and inhibit different RNases that don't share common sequences or active sites. This next section will outline the mechanism by which RI inhibits the catalytic properties of RNase 1.<br> |
<scene name='Ribonuclease_inhibitor/1z7x/3'>Reload Model</scene> <br> | <scene name='Ribonuclease_inhibitor/1z7x/3'>Reload Model</scene> <br> | ||
| - | First, examine the <scene name='Ribonuclease_inhibitor/Activesitecit/16'>active site</scene> of RNase 1. His12, Lys41, and His119 are three key catalytic residues to take note of. | + | First, examine the <scene name='Ribonuclease_inhibitor/Activesitecit/16'>active site</scene> of RNase 1. His12, Lys41, and His119 are three key catalytic residues to take note of. They have been colored CPK and represented in wireframe.<ref> PMID:9000628 </ref> <scene name='Ribonuclease_inhibitor/Activesitecit/17'>Human RI is ligated to a molecule of citric acid.</scene> Tyr434, Asp435, Ser460 form hydrogen bonds to the citrate.<scene name='Ribonuclease_inhibitor/Activesitecit/22'> Next,</scene> the citrate forms hydrogen bonds with His12, Lys41, and His119 of RNase 1. The binding of citrate interferes with the substrate-binding pocket of RNase 1 severely affecting its enzymatic ability. <ref> PMID:9000628 </ref> |
| - | {{Link Toggle Ribonuclease inhibitor/ | + | <br> |
| - | + | {{Link Toggle Ribonuclease inhibitor/chains}}<br> | |
| + | {{Link Toggle Ribonuclease inhibitor/Citrate}} | ||
| - | </structuresection> | ||
==Medical Implications== | ==Medical Implications== | ||
| - | As mentioned earlier in the introduction, ribonucleases are cytotoxic. They bind to and chop up RNA. RNase is an endonuclease and is diffusion limited, meaning it acts as fast as susbstrates arrive. RNases exhibit great stability and are often purified by sulfuric acid treatment and then boiling until it is the only surviving macromolecule. RI’s exist to protect cells from rogue RNases. <ref>http://www.pdb.org/pdb/101/motm.do?momID=105</ref> <br> <br> | + | As mentioned earlier in the introduction, ribonucleases are cytotoxic. They bind to and chop up RNA. RNase is an endonuclease and is diffusion limited, meaning it acts as fast as susbstrates arrive. RNases exhibit great stability and are often purified by sulfuric acid treatment and then boiling until it is the only surviving macromolecule. RI’s exist to protect cells from rogue RNases. <ref>http://www.pdb.org/pdb/101/motm.do?momID=105</ref> <br> <br> The amphibian RNase ONC is currently in clinical trials, however, human ribonucleases possess advantages over amphibian, namely increased catalytic ability, decreased renal toxicity, and decreased immunogenicity. The RI evasion of an RNase is critical to its chemotherapeutic effectiveness. Genetic engineers have been working on site-specific mutations that either decrease the association constant or increase the disassociation constant. |
| + | There are many interactions between hRI and RNase 1 providing a multitude of mutagenic options. However, it must be remembered that while changing residues can lead to increased RI evasion, because protein sequence determines structure, and structure determines form changing residues can also lead to decreased catalytic activity. Therefore, altering residues in exchange for RI evasion is a double-edged sword because cytotoxicity must be preserved. It's medically relevant to first understand the interactions between hRI and ribonucleases. Then one can design/modify a ribonuclease to be able to evade hRI while still maintaining functionality—potentially a new way to fight cancer. Researchers at the University of Wisconsin have been working on this very thing. One particular species, “R39D/N67D/N88A/ G89D/R91D RNase 1" has a 5×10^9-fold decrease in affinity for RI, while maintaing nearly wild-type ribonucleolytic activity, conformational stability, and cytotoxicity. <ref>PMID:17350650</ref> The R39D and R91D substitutions sever the hydrogen bonds formed by <scene name='Ribonuclease_inhibitor/Arg91/5'>Arg 91</scene> and <scene name='Ribonuclease_inhibitor/Arg39/1'>Arg 39</scene> that served as electrostatic targeting regions. The aspartates create negative/negative repulsion. This modified RNase provides an interesting example of the future direction and potential of biochemistry and medicine. | ||
| + | |||
| + | ==3D structures of RI== | ||
| + | |||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | |||
| + | [[1a4y]] – hRI + RNase 5 – human<br /> | ||
| + | [[2bex]] – hRI + nonsecretory RNase<br /> | ||
| + | [[1z7x]], [[2q4g]] - hRI + RNase I<br /> | ||
| + | [[4peq]] - bRI + RNase I - bovine<br /> | ||
| + | [[2bnh]] - pRI - pig<br /> | ||
| + | [[1dfj]] – pRI + bRNase I <br /> | ||
| + | [[3tsr]] – RI + RNAse I – mouse<br /> | ||
| + | [[4per]] - RI + RNase I - chicken<br /> | ||
| - | ==3d structures== {{STRUCTURE_1z7x| PDB=1z7x | SCENE= }} | ||
| - | '''RI·RNase Complexes''' <br> | ||
| - | [[1z7x]] Displayed right, hRI dimer complexed with two RNase 1 molecules <br> | ||
| - | [[1dfj]] -- Bovine RI + RNase A | ||
| - | <br>'''RI structures'''<br> | ||
| - | [[2bnh]] -- Porcine RI | ||
==References== | ==References== | ||
<references /> | <references /> | ||
| + | </structuresection> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Abe Weintraub, Michal Harel, Alexander Berchansky, Joel L. Sussman