Journal:Molecular Cell:1
From Proteopedia
(Difference between revisions)

| (9 intermediate revisions not shown.) | |||
| Line 9: | Line 9: | ||
The algorithm, '''PROSS''' (Protein Repair One-Stop Shop), is available at [http://pross.weizmann.ac.il http://pross.weizmann.ac.il ]. | The algorithm, '''PROSS''' (Protein Repair One-Stop Shop), is available at [http://pross.weizmann.ac.il http://pross.weizmann.ac.il ]. | ||
| - | <scene name='72/728277/Cv/ | + | <scene name='72/728277/Cv/41'>The structural basis underpinnings the stabilization in the designed variant dAChE4</scene>. <font color='slateblue'><b> Wild type hAChE (PDB entry: [[4ey4]]) is shown in blue</b></font> and <font color='darkorange'><b>51 mutated positions, which are distributed throughout dAChE4, are indicated by orange spheres</b></font>. |
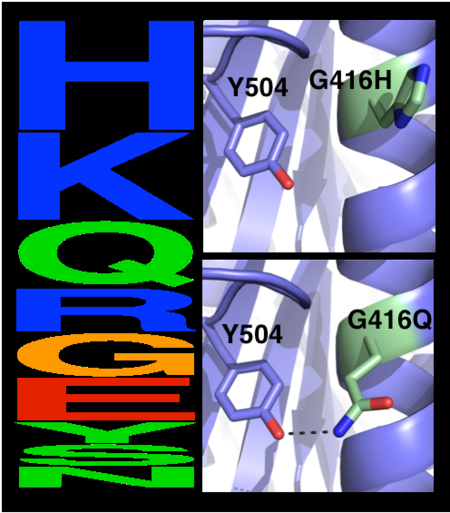
The choice of mutations at Gly416 in hAChE illustrates the role of the two filters, | The choice of mutations at Gly416 in hAChE illustrates the role of the two filters, | ||
| Line 37: | Line 37: | ||
*<scene name='72/728277/Cv/39'>Comparison of designed buried hydrogen bonds</scene>. Val331Asn was predicted to form a hydrogen bond with Glu450 and another with Pro446 in the designed model; in the crystal structure, instead, Asn331 interacts with Glu334 and Glu450. | *<scene name='72/728277/Cv/39'>Comparison of designed buried hydrogen bonds</scene>. Val331Asn was predicted to form a hydrogen bond with Glu450 and another with Pro446 in the designed model; in the crystal structure, instead, Asn331 interacts with Glu334 and Glu450. | ||
*<scene name='72/728277/Cv/40'>Leu394Asn forms 2 hydrogen bonds with Pro388 and Asp390, as designed</scene>. | *<scene name='72/728277/Cv/40'>Leu394Asn forms 2 hydrogen bonds with Pro388 and Asp390, as designed</scene>. | ||
| + | |||
| + | '''PDB reference:''' Stable, high-expression variant of human acetylcholinesterase, [[5hq3]]. | ||
</StructureSection> | </StructureSection> | ||
<references/> | <references/> | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
Current revision
| |||||||||||
- ↑ Goldenzweig A, Goldsmith M, Hill SE, Gertman O, Laurino P, Ashani Y, Dym O, Unger T, Albeck S, Prilusky J, Lieberman RL, Aharoni A, Silman I, Sussman JL, Tawfik DS, Fleishman SJ. Automated Structure- and Sequence-Based Design of Proteins for High Bacterial Expression and Stability. Mol Cell. 2016 Jul 21;63(2):337-346. doi: 10.1016/j.molcel.2016.06.012. Epub 2016, Jul 14. PMID:27425410 doi:http://dx.doi.org/10.1016/j.molcel.2016.06.012
Proteopedia Page Contributors and Editors (what is this?)
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.