Gamma secretase
From Proteopedia
(Difference between revisions)
(Category:Featured in BAMBED) |
|||
| (12 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == Gamma Secretase Interaction In Alzheimer's Disease == | + | {{BAMBED |
| - | <scene name='81/812869/Matilda_scene_5/1'>Gamma-secretase (GS)</scene> is a multi-subunit protease complex which cleaves many transmembrane proteins; it is known as an intramembrane protease. γ-secretase is highly studied in its cleavage of amyloid precursor protein (APP) releasing beta-amyloid (Aβ peptides) which further oligomerize to form neurofibrillary tangles and plaques in Alzheimer’s disease.<ref name= "mckeon">DOI: 10.1016/B978-012351830-9/50024-X</ref> | + | |DATE=December 3, 2020 |
| - | + | |OLDID=3326284 | |
| - | + | |BAMBEDDOI=10.1002/bmb.21560 | |
| - | + | }} | |
| + | |||
| + | __NoTOC__== Gamma Secretase Interaction In Alzheimer's Disease == | ||
| + | |||
| + | <StructureSection load='5A63' size='340' side='right' caption='Cryo EM structure of glycosylated Gamma Secretase Complex; Nicastrin (grey), presenilin (green), γ secretase subunit APH-1A (pink), γ secretase subunit PEN-2 (yellow) (PDB code [[5a63]])' scene=''> | ||
| + | <scene name='81/812869/Matilda_scene_5/1'>Gamma-secretase (GS)</scene> is a multi-subunit protease complex which cleaves many transmembrane proteins; it is known as an intramembrane protease. γ-secretase is highly studied in its cleavage of amyloid precursor protein (APP) releasing beta-amyloid (Aβ peptides) which further oligomerize to form neurofibrillary tangles and plaques in Alzheimer’s disease.<ref name= "mckeon">DOI:10.1016/B978-012351830-9/50024-X</ref> | ||
== Background == | == Background == | ||
The family of intramembrane-cleaving proteases (i-CLiPs) contains the presenilin family of '''aspartyl proteases''', zinc metalloprotease, site-2 protease family, rhomboid family of serine proteases, and γ-secretase. All i-CLiPs enzymatically cleave their substrates within the plane of the lipid bilayer in a process termed regulated intramembrane proteolysis. A large function of γ-secretase is its involvement in intramembranous proteolysis of type I membrane proteins. It cleaves numerous functionally important proteins, such as Notch, E-cadherin, ErbB4, CD44, tyrosinase, TREM2 and Alcadein, suggesting the participation of γ-secretase in a vast range of biological activities. The best-studied γ-secretase substrates are APP for its roles in Alzheimer’s Disease, and Notch for its importance in development and cell fate determination.<ref name= "zhang">DOI:10.3389/fncel.2014.00427</ref> | The family of intramembrane-cleaving proteases (i-CLiPs) contains the presenilin family of '''aspartyl proteases''', zinc metalloprotease, site-2 protease family, rhomboid family of serine proteases, and γ-secretase. All i-CLiPs enzymatically cleave their substrates within the plane of the lipid bilayer in a process termed regulated intramembrane proteolysis. A large function of γ-secretase is its involvement in intramembranous proteolysis of type I membrane proteins. It cleaves numerous functionally important proteins, such as Notch, E-cadherin, ErbB4, CD44, tyrosinase, TREM2 and Alcadein, suggesting the participation of γ-secretase in a vast range of biological activities. The best-studied γ-secretase substrates are APP for its roles in Alzheimer’s Disease, and Notch for its importance in development and cell fate determination.<ref name= "zhang">DOI:10.3389/fncel.2014.00427</ref> | ||
| + | |||
| + | GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments.<ref name="Bolduc" /> Products of initial APP cleavage include the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40. [https://en.wikipedia.org/wiki/Amyloid_beta Aβ products] are connected to neurological diseases such as [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's disease (AD)], with varying length peptide products showing different disease symptoms. The connection between GS and AD has made GS a popular drug target. Although various inhibitors of GS have been identified, no inhibitors have been clinically approved for treating AD, as GS is also linked to important neurological functions and inhibition of these GS functions leads to dangerous side effects upon inhibition.<ref name="Zhou">PMID:30630874</ref> | ||
== Structure of Gamma Secretase Complex == | == Structure of Gamma Secretase Complex == | ||
| - | γ-secretase has been identified as an aspartyl protease accountable for cleaving over 90 integral membrane proteins after they have undergone ectodomain shedding. GS has been characterized as a high molecular weight complex that consists of four essential subunits in a 1:1:1:1 heterodimer<ref name= "carroll">DOI:10.1016/j.brainresbull.2016.04.019</ref>: <scene name='81/812869/Matilda_scene_2/12'>Presenilin (PS, PS1, or PS2)</scene>, <scene name='81/812869/Matilda_scene_1/13'>Nicastrin (NCT)</scene>, <scene name='81/812869/Matilda_scene_3/2'>Anterior Pharynx-Defective-1 (APH-1)</scene>, and <scene name='81/812869/Matilda_scene_4/4'>Presenilin Enhancer-2 (PEN-2)</scene>.<ref name= "thompson">DOI:10.1186/1756-6606-4-3</ref> | + | γ-secretase has been identified as an aspartyl protease accountable for cleaving over 90 integral membrane proteins after they have undergone ectodomain shedding. GS has been characterized as a high molecular weight complex that consists of four essential subunits in a 1:1:1:1 heterodimer<ref name= "carroll">DOI:10.1016/j.brainresbull.2016.04.019</ref>: <scene name='81/812869/Matilda_scene_2/12'>Presenilin (PS, PS1, or PS2)</scene>, <scene name='81/812869/Matilda_scene_1/13'>Nicastrin (NCT)</scene>, <scene name='81/812869/Matilda_scene_3/2'>Anterior Pharynx-Defective-1 (APH-1)</scene>, and <scene name='81/812869/Matilda_scene_4/4'>Presenilin Enhancer-2 (PEN-2)</scene>.<ref name= "thompson">DOI:10.1186/1756-6606-4-3</ref>These subunits are stabilized as functional GS by hydrophobic interactions and 4 [https://en.wikipedia.org/wiki/Phosphatidylcholine phosphatidylcholines].These <scene name='83/832945/Phosphotidylcholines/2'>phosphatidylcholines</scene> have interfaces between: PS1 and PEN-2, APH-1 and PS1, APH-1 and NCT. |
| + | |||
PSs play a very significant role in AD and is considered a vital catalytic subunit in γ-secretase. PS are multi-transmembrane proteins with nine transmembrane helixes; it is assumed the amino-terminus is located in the cytosol while the carboxyl-terminus is exposed to the luminal/extracellular space. Functional PS requires endoproteolytic cleavage between TM6 and TM7 which generates a 27–28 kDa amino-terminal fragment (NTF) and a 16–17 kDa carboxyl-terminal fragment (CTF). <ref name="zhang" /> The two <scene name='81/812869/Matilda_scene_10/1'>aspartyl residues</scene> in PS1 and PS2 ('''D257''' in TM 6 and at '''D385''' in TM 7) play crucial roles in intramembranous cleavage and AD plaque formation; substitutions of these residues reduces cleavage of APP and Notch1 proteins.<ref name= "o'brien">DOI:10.1146/annurev-neuro-061010-113613</ref> PS, NTF, and CTF bind to form stable and active PS heterodimers at a 1:1 stoichiometry. <ref name="zhang" /> | PSs play a very significant role in AD and is considered a vital catalytic subunit in γ-secretase. PS are multi-transmembrane proteins with nine transmembrane helixes; it is assumed the amino-terminus is located in the cytosol while the carboxyl-terminus is exposed to the luminal/extracellular space. Functional PS requires endoproteolytic cleavage between TM6 and TM7 which generates a 27–28 kDa amino-terminal fragment (NTF) and a 16–17 kDa carboxyl-terminal fragment (CTF). <ref name="zhang" /> The two <scene name='81/812869/Matilda_scene_10/1'>aspartyl residues</scene> in PS1 and PS2 ('''D257''' in TM 6 and at '''D385''' in TM 7) play crucial roles in intramembranous cleavage and AD plaque formation; substitutions of these residues reduces cleavage of APP and Notch1 proteins.<ref name= "o'brien">DOI:10.1146/annurev-neuro-061010-113613</ref> PS, NTF, and CTF bind to form stable and active PS heterodimers at a 1:1 stoichiometry. <ref name="zhang" /> | ||
| - | The remaining <scene name='81/812869/Matilda_scene_9/1'>three subunits</scene> (NCT, APH-1, PEN-2) help with stabilizing GS by forming a mature enzyme. NCT contains a large extracellular (or ectodomain) domain, transmembrane helix, and smaller cytoplasmic domain.<ref name= "carroll" />The ectodomain of NCT recognizes and binds to the amino-terminal stubs of previously cleaved transmembrane proteins. APH-1 aids the formation of a pre-complex, which interacts with PS1 or PS2<ref name= "o'brien" />; it contains two different isoforms from two paralogous genes on chromosomes 1 '''(APH-1A)''' and 15 '''(APH-1B)'''. While PEN-2 works in enzyme maturation<ref name= "carroll" />; it enters the formed complex to initiate the cleavage of PS1 or PS2 to form an N-terminal 28-kDa fragment and a C-terminal 18-kDa fragment, both APH-1 and PEN-2 are critical to the γ-secretase complex.<ref name= "o'brien" /> The γ-secretase complex has a molecular weight of approximately 170 kDa, with an additional 30–70 kDa derived from NCT glycosylation, reaching a total size of about 230 kDa with 19 TMs.<ref name= "zhang" /> | + | The remaining <scene name='81/812869/Matilda_scene_9/1'>three subunits</scene> (NCT, APH-1, PEN-2) help with stabilizing GS by forming a mature enzyme. NCT contains a large extracellular (or ectodomain) domain, transmembrane helix, and smaller cytoplasmic domain.<ref name= "carroll" />The ectodomain of NCT recognizes and binds to the amino-terminal stubs of previously cleaved transmembrane proteins. <scene name='83/832945/Nct_subunit_shown/1'>NCT</scene> is important to substrate recognition and binding. APH-1 aids the formation of a pre-complex, which interacts with PS1 or PS2<ref name= "o'brien" />; it contains two different isoforms from two paralogous genes on chromosomes 1 '''(APH-1A)''' and 15 '''(APH-1B)'''. <scene name='83/832945/Aph-1_subunit/1'>APH-1</scene> also serves as a scaffold for anchoring and supporting the flexible conformational changes of PS1. While PEN-2 works in enzyme maturation<ref name= "carroll" />; it enters the formed complex to initiate the cleavage of PS1 or PS2 to form an N-terminal 28-kDa fragment and a C-terminal 18-kDa fragment, both APH-1 and PEN-2 are critical to the γ-secretase complex.<ref name= "o'brien" /> Activation of the active site is dependent on the binding of <scene name='83/832945/Pen2_subunit/1'>PEN-2</scene>. PEN-2 is also important in maturation of the enzyme.<ref name="Yang">PMID:28628788</ref> The γ-secretase complex has a molecular weight of approximately 170 kDa, with an additional 30–70 kDa derived from NCT glycosylation, reaching a total size of about 230 kDa with 19 TMs.<ref name= "zhang" /> |
== Function == | == Function == | ||
| Line 37: | Line 45: | ||
The structural information of the γ-secretase complex has been primarily obtained by electron microscopy analysis with a maximum resolution of 12 Å, revealing a <scene name='81/812869/Matilda_scene_5/2'>globular structure</scene> with several extracellular domains, three <scene name='81/812869/Matilda_scene_5/3'>polar</scene> cavities, and a potential substrate-binding surface groove in the TM region. The most recent sturcutre of the γ-secretase complex was visualized by a cryo-electron microscopy with a resolution of 4.5 Å. γ-secretase exhibits a horseshoe-shaped structure with 19 TMs and a bilobed ectodomain which is Nicastrin, "the extracellular domain of Nicastrin contains a large lobe and a small lobe." The large lobe of Nicastrin thought to be responsible for substrate recognition, associates with the small lobe through a <scene name='81/812869/Matilda_scene_6/1'>hydrophobic pivot</scene> at the center. The horseshoe shape is described as having a "thick" end, where PS1 and PEN-2 are located, and "thin" end, where APH-1 and Nicastrin are located. At the thick end, PEN-2 spans the membrane twice as its N- and C-terminal domains face the lumen of the ER. However, at the thin end, APH-1 contains seven TM domains with the N-terminal domain facing the extracellular space and the C-terminal domain facing the cytosol. Further work is required to elucidate structural details of other γ-secretase components at the atomic level.<ref name= "zhang" /> However, strong evidence suggests that the γ-secretase complex resides primarily in the ER, Golgi/TGN, endocytic and intermediate compartments, most of which (except the TGN) are not major subcellular localizations for APP.<ref name="thompson" /> | The structural information of the γ-secretase complex has been primarily obtained by electron microscopy analysis with a maximum resolution of 12 Å, revealing a <scene name='81/812869/Matilda_scene_5/2'>globular structure</scene> with several extracellular domains, three <scene name='81/812869/Matilda_scene_5/3'>polar</scene> cavities, and a potential substrate-binding surface groove in the TM region. The most recent sturcutre of the γ-secretase complex was visualized by a cryo-electron microscopy with a resolution of 4.5 Å. γ-secretase exhibits a horseshoe-shaped structure with 19 TMs and a bilobed ectodomain which is Nicastrin, "the extracellular domain of Nicastrin contains a large lobe and a small lobe." The large lobe of Nicastrin thought to be responsible for substrate recognition, associates with the small lobe through a <scene name='81/812869/Matilda_scene_6/1'>hydrophobic pivot</scene> at the center. The horseshoe shape is described as having a "thick" end, where PS1 and PEN-2 are located, and "thin" end, where APH-1 and Nicastrin are located. At the thick end, PEN-2 spans the membrane twice as its N- and C-terminal domains face the lumen of the ER. However, at the thin end, APH-1 contains seven TM domains with the N-terminal domain facing the extracellular space and the C-terminal domain facing the cytosol. Further work is required to elucidate structural details of other γ-secretase components at the atomic level.<ref name= "zhang" /> However, strong evidence suggests that the γ-secretase complex resides primarily in the ER, Golgi/TGN, endocytic and intermediate compartments, most of which (except the TGN) are not major subcellular localizations for APP.<ref name="thompson" /> | ||
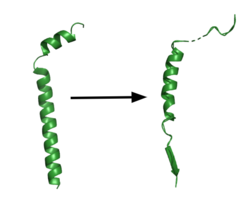
| - | This is a | + | ===Substrate Structure=== |
| + | [[Image:App.png|250 px|right|thumb|'''Figure 1. APP fragment conformational change in gamma secretase.''' APP bound to GS undergoes a conformational change. The free state consists of 2 helices. Once bound to GS, the N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. The β-strand of APP forms a β-sheet with PS1. Cleavage by the protease occurs between the helix and the β-strand.<ref name= "Zhou" />]] | ||
| + | GS has been structurally characterized in the presence of both [https://en.wikipedia.org/wiki/Amyloid_precursor_protein/ APP] and Notch substrates. In each of these structures, the substrate bound in a similar location and underwent a similar structural transition upon binding to the active site of GS. Each substrate is composed of an N-terminal loop and a TM helix. The peptide substrate enters the enzyme by <scene name='83/832945/App_in_gs_general/2'>lateral diffusion</scene> via the lid complex, and once in place, the TM helix of the substrate is anchored by <scene name='83/832945/Hydrophobic_interactions/2'>van der Waals contacts</scene>. Upon binding to GS, the C-terminal extracellular helix of the substrate unwinds. The substrate's N-terminal end of the TM helix unwinds into a β-strand (Fig. 1). To differentiate substrates, the β-strand is often the main point of identification for the enzyme. Substrate binding induces a structural change in GS, creating two β-strands that form a β-sheet with the one β-strand of the substrate. This β-sheet is in close proximity with the active site, and guides the process of catalysis.<ref name="Zhou">PMID:30630874</ref> | ||
| + | |||
| + | ===Lid Complex=== | ||
| + | The <scene name='83/832945/Global_lid2/1'>lid complex</scene> is the first point of entry and recognition for the substrate. <scene name='83/832945/Lidremake2/2'>The lid</scene> is located within the NCT subunit between Asn55 and Asn435. This lobe of NCT is divided into two separate subunits; the large and small lobes with Phe287 from the large lobe acting as a pivot between them. Phe287 is surrounded by <scene name='83/832945/Pivot3/1'>Phe103, Leu171, Phe176, and Ile180</scene> of the small subunit. The congregation of hydrophobic residues in the small subunit composes a greasy pocket which provides an environment for easy structural movement. The lid consists of 5 aromatic residues, which are highly involved with stabilizing the closed conformation. This conformation is stabilized by <scene name='83/832945/Trp164scene/2'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic binding pocket is revealed. The pocket contains <scene name='83/832945/Gluandtyr_remake2/1'>Glu333 and Tyr337 surrounded by several charged residues</scene>. The pocket is further involved with substrate binding and recognition once the lid is removed. The lid complex is relatively far away from the catalytic site of the enzyme in PS1 when inactive. Once a substrate binds, the enzyme undergoes a conformational change in which the rotation of the large lobe in relation to the small lobe reorients the substrate for cleavage, by aligning the pocket in NCT to the active site in PS1.<ref name="Bai">PMID:26280335</ref> | ||
| + | |||
| + | ===Active Site=== | ||
| + | The <scene name='83/832945/Asp_257_and_asp_385/13'>active site</scene> is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Both TM6 and TM7 contribute an aspartate residue to the active site. These two aspartates, Asp257 and Asp385 are located approximately 10.6 A˚ apart when inactive.<ref name="Bai">PMID:26280335</ref> Substrate recognition is controlled by the closely spaced PAL sequence of <scene name='83/832945/Asp_257_and_asp_385/11'>Pro433, Ala434, and Leu435</scene>. GS becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate. Two β-strands are induced in PS1, creating an <scene name='83/832945/Beta_sheet_complex/1'>antiparallel β-sheet</scene> with the β-strand of the substrate.<ref name="Zhou" /> The β-strand of the substrate interacts via main chain H-bonds <scene name='83/832945/Pal_and_app/1'>with the PAL sequence</scene>, stabilizing the active site. <scene name='83/832945/Asp_257_and_asp_385/10'>Asp257 and Asp385</scene> hydrogen bond to each other and are located 6–7 Å away from the scissile peptide bond of the substrate, allowing catalysis to occur.<ref name="Yang" /> GS cleaves in 3 residue segments which is driven by the presence of three amino acid binding pockets in the active site.<ref name="Bolduc" /> | ||
| + | In APP, the cleavage site is between the helix and the N-terminal β-strand.<ref name="Zhou" /> GS can cleave via different pathways, depending on its starting point, but the 2 most commonly used pathways produce Aβ48 and Aβ49.<ref name="Bolduc">PMID:27580372</ref>. Tripeptide cleavage starting between <scene name='83/832945/3_residues_for_cleavage/2'>Thr719 and Leu720</scene> results in Aβ48. Cleavage between <scene name='83/832945/3_residues_for_cleavage/3'>Leu720 and Val721</scene> yields Aβ49. The accumulation of these Aβ peptides has strong implications in Alzheimer's disease.<ref name="Zhou">PMID:30630874</ref> | ||
| + | |||
| + | ==3D structures of γ secretase== | ||
| + | |||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | [[4r12]] – hGS nicastrin component<br /> | ||
| + | [[2n7q]], [[2n7r]] – hGS nicastrin component transmembrane domain - NMR<br /> | ||
| + | [[5a63]], [[5fn5]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin – human – Cryo EM<br /> | ||
| + | [[4uis]] – hGS + lysozyme – Cryo EM<br /> | ||
| + | [[8im7]], [[8oqy]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin – Cryo EM<br /> | ||
| + | [[6iyc]], [[8k8e]], [[8oqz]], [[8x52]], [[8x53]], [[8x54]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin + amyloid-beta A4 protein – Cryo EM<br /> | ||
| + | [[5fn3]], [[5fn4]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin + poly-Ala – Cryo EM<br /> | ||
| + | [[6idf]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin + notch 1 – Cryo EM<br /> | ||
| + | [[5fn2]], [[8kco]], [[8kcp]], [[8kcs]], [[8kct]], [[8kcu]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin + drug – Cryo EM<br /> | ||
| + | [[6lqg]], [[6lr4]], [[7c9i]], [[7d8x]], [[7y5t]], [[7y5x]], [[7y5z]] – hGS APH-1A+PEN-2 subunits + nicastrin + presenilin + inhibitor – Cryo EM<br /> | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | </StructureSection> | ||
| + | __NoTOC__ | ||
| + | |||
| + | ==Student Contributors== | ||
| + | Daniel Mulawa | ||
| + | |||
| + | Layla Wisser | ||
| + | |||
| + | [[Category:Topic Page]] | ||
| + | [[Category:Featured in BAMBED]] | ||
Current revision
This page, as it appeared on December 3, 2020, was featured in this article in the journal Biochemistry and Molecular Biology Education.
Gamma Secretase Interaction In Alzheimer's Disease
| |||||||||||
Student Contributors
Daniel Mulawa
Layla Wisser
Proteopedia Page Contributors and Editors (what is this?)
Matilda Dervisevic, Michal Harel, R. Jeremy Johnson, Angel Herraez, Jaime Prilusky