We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Virus protease
From Proteopedia
(Difference between revisions)
| (69 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='' size='350' side='right' caption='Structure of human rhinovirus 3C protease complex with inhibitor (PDB entry [[1cqq]])' scene='55/554890/Cv/1'> | |
| - | + | __TOC__ | |
| + | == Function == | ||
| + | |||
| + | '''Virus protease''' or '''3C protease''' or '''virus proteinase''' are virus cysteine proteases which have essential role in viral replication. The protease cleaves the precursor viral polyprotein to produce functional proteins and enzymes.<ref>PMID:17305557</ref> F | ||
| - | + | == Disease == | |
| - | + | ||
| + | Virus proteases are attractive target for therapeutic agents for treatment of diseases caused by the common cold virus. Coxsakievirus causes the human diseases myocarditis and pericarditis. | ||
| - | + | == Structural highlights == | |
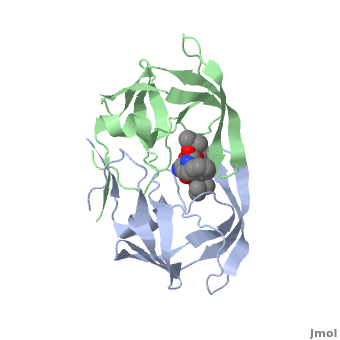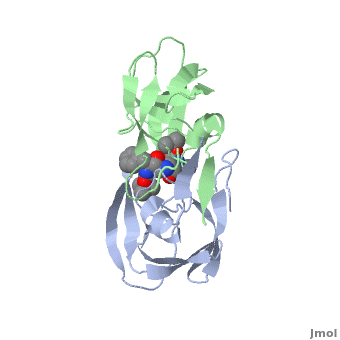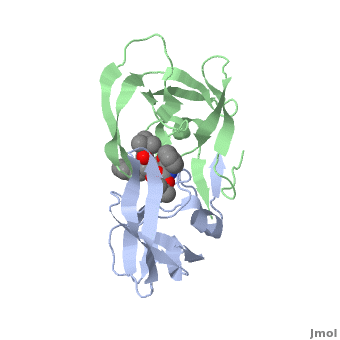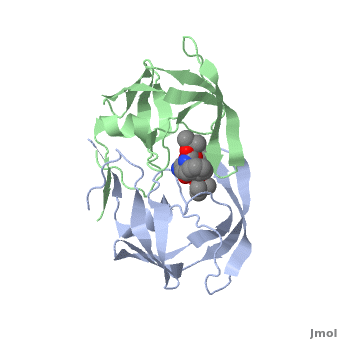
| + | Viral protease - a Cys protease fold is similar to that of trypsin-like serine proteases. The <scene name='55/554890/Cv/6'>active site triad contains Cys147, His40 and Glu71</scene> (PDB entry [[1cqq]]).<ref>PMID:10500114</ref> <scene name='55/554890/Cv/5'>Whole binding site</scene>. | ||
| + | See also<br /> | ||
| + | *[[Immunodeficiency virus protease]]<br /> | ||
| + | *[[Human Immunodeficiency Virus]]<br /> | ||
| + | *[[HIV-1 protease]]<br /> | ||
| + | *[[HIV Protease Inhibitor Pharmacokinetics]]<br /> | ||
| + | *[[HIV Protease Inhibitor Resistance Profile]]<br /> | ||
| + | *[[HIV Protease Resistance]] <br /> | ||
| + | *[[Molecular Playground/Dengue Virus Protease]]<br /> | ||
| + | *[[Viability of a drug-resistant HIV-1 protease mutant]]<br /> | ||
| + | *[[FMDV 3C]] for Foot-and-Mouth virus protease<br /> | ||
| + | *[[SARS Coronavirus Main Proteinase]]<br /> | ||
| + | *[[SARS-CoV-2 Coronavirus Main Protease]]<br /> | ||
| + | *[[Taylor SARS-CoV2 Protease]]<br /> | ||
| + | == 3D Structures of Virus protease == | ||
| + | [[Virus protease 3D structures]] | ||
| + | </StructureSection> | ||
| - | + | ==References== | |
| - | == | + | <references /> |
| - | + | [[Category:Topic Page]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[ | + | |
Current revision
| |||||||||||
References
- ↑ Wanga QM, Chen SH. Human rhinovirus 3C protease as a potential target for the development of antiviral agents. Curr Protein Pept Sci. 2007 Feb;8(1):19-27. PMID:17305557
- ↑ Matthews DA, Dragovich PS, Webber SE, Fuhrman SA, Patick AK, Zalman LS, Hendrickson TF, Love RA, Prins TJ, Marakovits JT, Zhou R, Tikhe J, Ford CE, Meador JW, Ferre RA, Brown EL, Binford SL, Brothers MA, DeLisle DM, Worland ST. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):11000-7. PMID:10500114
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky