RNase A Oligomers
From Proteopedia
(Difference between revisions)
(adding BAMBED ref) |
|||
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | = | + | {{BAMBED |
| + | |DATE=May 26, 2011 | ||
| + | |OLDID=1249782 | ||
| + | |BAMBEDDOI=10.1002/bmb.20568 | ||
| + | }} | ||
| + | <StructureSection load='' size='450' side='right' scene='Sandbox_Reserved_200/Minor_dimer/4' caption=''> | ||
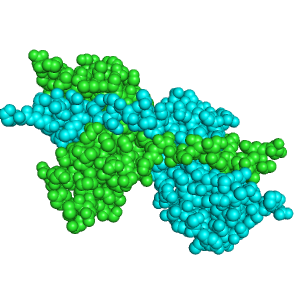
| - | + | [[Image:2D_RNaseA.png|300px|left|thumb|RNase A minor dimer, [[1a2w]]]] | |
| - | [[Image:2D_RNaseA.png|300px|left|thumb|RNase A minor dimer, [[ | + | Bovine pancreatic '''ribonuclease A (RNase A)''' is an enzyme that catalyzes the hydrolysis of RNA through [http://www.proteopedia.org/wiki/index.php/Sandbox_Reserved_193 acid-base catalysis]. RNase A has the capability to structurally form dimers, trimers, tetramers, and pentamers based on the structure of the [http://www.proteopedia.org/wiki/index.php/Sandbox_Reserved_192 RNase A monomer]. Though there are many oligomers, the three-dimensional structure for only the major dimer, minor dimer, and minor trimer are known. Unlike the monomers, all the oligomers are capable of catalyzing the hydrolysis of double stranded RNA (dsRNA).<ref name="tumor">PMID:12697760</ref> The oligomers are formed by 3D domain swapping, which can occur once or twice per monomeric unit <ref name="liul">PMID:11224563</ref >. The 3D domain swapping has no impact on the formation of active sites which is the same in the monomers and all oligomers.<ref name="liul"/> The oligomers of RNase A also show medical relevance as [[Pharmaceutical Drugs|antitumor drugs]] as well as models to understand the possible cause of [[Alzheimer's Disease]]. |
| - | Bovine pancreatic ribonuclease A | + | |
==Dimers== | ==Dimers== | ||
| - | + | ||
Ribonuclease A has both a <scene name='Sandbox_Reserved_200/Major_dimer/11'>major</scene> and <scene name='Sandbox_Reserved_200/Minor_dimer/4'>minor</scene> dimer which are very similar to one another. Though they are similar, they are formed by different types of 3D domain swapping. 3D domain swapping occurs when identical domains are interchanged. The major dimer is formed by 3D domain swapping the β-strand of the C-terminus.<ref name="liu98"/> The minor dimer, on the other hand, is formed by 3D domain swapping of the α-helix on the N-terminus <ref name="liu98"/>. Domain swapping is extremely specific and can only occur at the <scene name='Sandbox_Reserved_200/Major_dimer/10'>C-terminus</scene> or the <scene name='Sandbox_Reserved_200/Major_dimer/9'>N-terminus</scene>. | Ribonuclease A has both a <scene name='Sandbox_Reserved_200/Major_dimer/11'>major</scene> and <scene name='Sandbox_Reserved_200/Minor_dimer/4'>minor</scene> dimer which are very similar to one another. Though they are similar, they are formed by different types of 3D domain swapping. 3D domain swapping occurs when identical domains are interchanged. The major dimer is formed by 3D domain swapping the β-strand of the C-terminus.<ref name="liu98"/> The minor dimer, on the other hand, is formed by 3D domain swapping of the α-helix on the N-terminus <ref name="liu98"/>. Domain swapping is extremely specific and can only occur at the <scene name='Sandbox_Reserved_200/Major_dimer/10'>C-terminus</scene> or the <scene name='Sandbox_Reserved_200/Major_dimer/9'>N-terminus</scene>. | ||
| Line 15: | Line 19: | ||
Not only is the structure of the monomers conserved in the dimers, but the active is also conserved. <ref name="liu98"/> The <scene name='Sandbox_Reserved_200/Minor_dimer/9'>active site</scene> of both dimers contains His12, Lys41, and His119 residues. The active sites are a composite of the monomer subunits containing <scene name='Sandbox_Reserved_200/Minor_dimer/7'>His 12</scene> from one monomer and His119 form the other monomer.<ref name="liul"/> During domain swapping, the active site is not disturbed, so the dimers are able to retain their enzymatic activity. | Not only is the structure of the monomers conserved in the dimers, but the active is also conserved. <ref name="liu98"/> The <scene name='Sandbox_Reserved_200/Minor_dimer/9'>active site</scene> of both dimers contains His12, Lys41, and His119 residues. The active sites are a composite of the monomer subunits containing <scene name='Sandbox_Reserved_200/Minor_dimer/7'>His 12</scene> from one monomer and His119 form the other monomer.<ref name="liul"/> During domain swapping, the active site is not disturbed, so the dimers are able to retain their enzymatic activity. | ||
| - | |||
| - | |||
==Trimers== | ==Trimers== | ||
RNase A trimers are formed in the same way as the dimers, except there are now three monomeric subunits. There is both a major and minor trimer. The structure of the major trimer is not known, but the two trimers can be separated by both chromatography and gel electrophoresis. <ref name="liu01"/> The major trimer is more common than the minor trimer. | RNase A trimers are formed in the same way as the dimers, except there are now three monomeric subunits. There is both a major and minor trimer. The structure of the major trimer is not known, but the two trimers can be separated by both chromatography and gel electrophoresis. <ref name="liu01"/> The major trimer is more common than the minor trimer. | ||
| - | <Structure load='1JS0' size='350' frame='true' align='left' caption='Minor Trimer of RNase A' scene='Sandbox_Reserved_200/Minor_trimer/12' /> | ||
| - | |||
The <scene name='Sandbox_Reserved_200/Minor_trimer/12'>minor trimer</scene> forms a cyclic propeller like shape. It is 3D domain swapped at the C-terminus of the beta strand.<ref name="liu01"/> No domain swapping a the N-terminus has been seen. When the minor trimer dissociates, it forms a dimer and a monomer. The minor and major dimer are both formed, but the major dimer is much more common.<ref name="liu01"/> | The <scene name='Sandbox_Reserved_200/Minor_trimer/12'>minor trimer</scene> forms a cyclic propeller like shape. It is 3D domain swapped at the C-terminus of the beta strand.<ref name="liu01"/> No domain swapping a the N-terminus has been seen. When the minor trimer dissociates, it forms a dimer and a monomer. The minor and major dimer are both formed, but the major dimer is much more common.<ref name="liu01"/> | ||
| Line 35: | Line 35: | ||
==Medical Relevance== | ==Medical Relevance== | ||
| - | [ | + | [[Alzheimer's Disease]] is a terminal disease that slowly degenerates the brain. One of the possible causes of Alzheimer’s is [http://en.wikipedia.org/wiki/Amyloid amyloid] deposits throughout the brain. Though RNase A oligomers are not the amyloid deposits that cause Alzheimer’s, the folding of these oligomers gives clues about the formation of the amyloid deposits responsible for Alzheimer’s. |
| Line 47: | Line 47: | ||
==Additional Proteopedia Pages about RNase A== | ==Additional Proteopedia Pages about RNase A== | ||
| - | * [http:// | + | * [http://www.proteopedia.org/wiki/index.php/RNase_A RNase A] |
| - | * [http:// | + | * [http://www.proteopedia.org/wiki/index.php/RNase_A_Oligomers RNase A oligomers] |
| - | * [http:// | + | * [http://www.proteopedia.org/wiki/index.php/RNase_A_NMR RNase A NMR] |
| - | * [http:// | + | * [http://www.proteopedia.org/wiki/index.php/RNaseS_RNaseB RNase S and RNase B] |
| + | * [http://www.proteopedia.org/wiki/index.php/RNaseA_Nobel_Prizes RNase A Nobel Prizes] | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
| + | |||
| + | |||
| + | ==3D structures of ribonuclease== | ||
| + | |||
| + | [[Ribonuclease]] | ||
==References== | ==References== | ||
| Line 67: | Line 75: | ||
*[http://www.proteopedia.org/wiki/index.php/User:Lexi_Gehring Lexi Gehring and Deanna Proimos] | *[http://www.proteopedia.org/wiki/index.php/User:Lexi_Gehring Lexi Gehring and Deanna Proimos] | ||
| + | |||
| + | [[Category:Featured in BAMBED]] | ||
Current revision
This page, as it appeared on May 26, 2011, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
3D structures of ribonuclease
References
- ↑ 1.0 1.1 1.2 1.3 Matousek J, Gotte G, Pouckova P, Soucek J, Slavik T, Vottariello F, Libonati M. Antitumor activity and other biological actions of oligomers of ribonuclease A. J Biol Chem. 2003 Jun 27;278(26):23817-22. Epub 2003 Apr 14. PMID:12697760 doi:10.1074/jbc.M302711200
- ↑ 2.0 2.1 2.2 2.3 Liu Y, Gotte G, Libonati M, Eisenberg D. A domain-swapped RNase A dimer with implications for amyloid formation. Nat Struct Biol. 2001 Mar;8(3):211-4. PMID:11224563 doi:10.1038/84941
- ↑ 3.0 3.1 3.2 3.3 3.4 Liu Y, Hart PJ, Schlunegger MP, Eisenberg D. The crystal structure of a 3D domain-swapped dimer of RNase A at a 2.1-A resolution. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3437-42. PMID:9520384
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Liu Y, Gotte G, Libonati M, Eisenberg D. Structures of the two 3D domain-swapped RNase A trimers. Protein Sci. 2002 Feb;11(2):371-80. PMID:11790847
- ↑ Libonati M, Gotte G. Oligomerization of bovine ribonuclease A: structural and functional features of its multimers. Biochem J. 2004 Jun 1;380(Pt 2):311-27. PMID:15104538 doi:10.1042/BJ20031922
External Resources
Student Contributors
Proteopedia Page Contributors and Editors (what is this?)
David Canner, R. Jeremy Johnson, Michal Harel, Alexander Berchansky, Angel Herraez, Jaime Prilusky