|
|
| (28 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
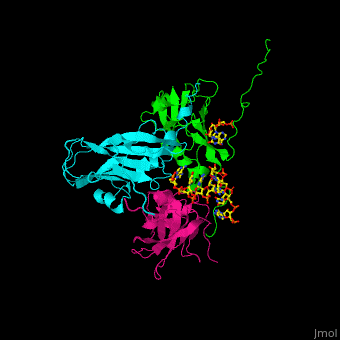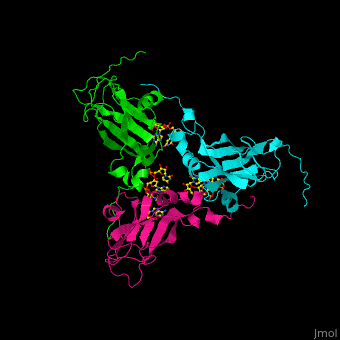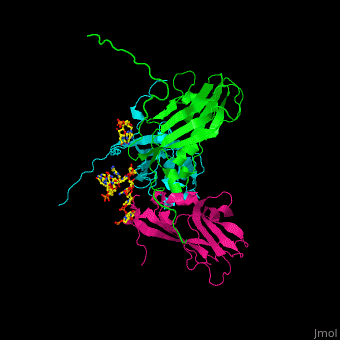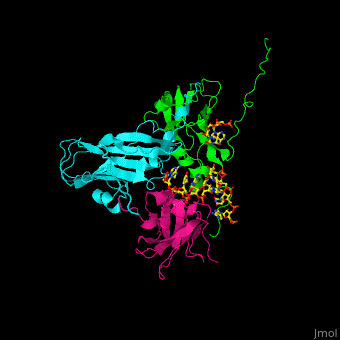
| - | <Structure load='1CWP' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | + | <StructureSection load='' size='350' side='right' caption='Cowpea chlorotic mottle virus coast protein trimer complex with RNA (PDB entry [[1cwp]])' scene='47/472643/Cv/1'> |
| | + | == Function == |
| | | | |
| - | ===Self-Assembling Cowpea Chlorotic Mottle Virus Capsid: Nanoreactor and Scaffold for Molecular Synthesis===
| + | '''Cowpea chlorotic mottle virus''' (CPMV) infects black-eyed pea plants. The CPMV is composed of protein shell (capsid) and RNA. |
| - | <scene name='Cowpea_Chlorotic_Mottle_Virus/Full_capsid/2'>Assembled Capsid</scene> | + | <scene name='47/472643/Cv/2'>Cowpea chlorotic mottle virus coast protein trimer with RNA</scene> (PDB entry [[1cwp]]). |
| | + | == Relevance == |
| | | | |
| | + | CPMV is used in nanotechnology due to its pH and metal ion-dependent polymorphism which can be used for delivery of small molecules. <ref>PMID:7743132</ref> |
| | + | </StructureSection> |
| | + | ==3D structures of CPMV== |
| | + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} |
| | | | |
| - | '''Introduction'''
| + | [[1ny7]], [[2bfu]], [[5ms1]], [[5msh]] – CPMV small + large subunits<br /> |
| | + | [[5a33]] – CPMV small + large subunits – Cryo EM<br /> |
| | + | [[6qoz]] – CPMV small + large subunits + affimer binding protein – Cryo EM<br /> |
| | + | [[1cwp]] – CPMV coat protein + RNA<br /> |
| | + | [[1za7]] – CPMV coat protein (mutant)<br /> |
| | | | |
| - | The cowpea chlorotic mottle virus is a plant virus that infects the cowpea plant. It is a naked (non-enveloped), icosahedral virus, and it contains a positive-strand RNA genome. Therefore, its encapsidated nucleic acids can be directly translated by host machinery to make the protein components for the assembly of progeny virions. Its replication strategy requires the formation of a negative-strand template that can be used to synthesize new positive-strand RNA.
| + | == References == |
| - | | + | <references/> |
| - | An important feature of virus is its ability to undergo structural changes under certain conditions without destroying the tertiary structure of its subunits. Specifically, when the environmental conditions are acidic (pH 5) and , the capsid is in a stable, assembled form. However, when the pH is raised ~6.5-7 with low ionic strength, the capsid undergoes a structural change called swelling.
| + | [[Category:Topic Page]] |
| - | During this process, the size of the virion increases by 10%. A comparative sedimentation will
| + | |
| - | This stage is considered to be an intermediate in the infection process, allowing genomic contents from within the capsid to come out of the capsid.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | <scene name='Cowpea_Chlorotic_Mottle_Virus/Isolated_rna/1'>Isolated RNA</scene>
| + | |
| - | | + | |
| - | | + | |
| - | '''General Capsid Structure'''
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | The viral capsid of CCMV is a complex of proteins stabilized by metal coordination between capsomeres and RNA binding on its internal surface.
| + | |
| - | <scene name='Cowpea_Chlorotic_Mottle_Virus/Protein_and_rna/1'>Protein Core with Stabilizing RNA Interactions</scene>
| + | |
| - | The viral genome encodes three capsid proteins that are chemically identical. These subunits, arbitrarily called A, B, and C, dimerize with each other and assemble into into hexamers and pentamers that comprise the viral capsomeres.<ref name=Speir>PMID:7743132</ref> The complete capsid structure takes the form of a truncated icosahedron (20 faces). It is described as a T=3 capsid, where the T value describes the number of structural units per equilateral face of the icosahedron.[http://www.nlv.ch/Virologytutorials/Structure.htm]
| + | |
| - | | + | |
| - | '''Capsomere Structure'''
| + | |
| - | | + | |
| - | The hexameric capsomeres are formed by the B and C subunits. The N-terminus arms (residues 27 through 49) of the subunits intertwine to form a parallel beta barrel.<ref name=Speir>PMID:7743132</ref> The N-terminus is therefore key to the hexamer composition.<ref name=Speir>PMID:7743132</ref>
| + | |
| - | <scene name='Cowpea_Chlorotic_Mottle_Virus/Residues_27-49/1'>N-terminus arms</scene>
| + | |
| - | The atoms of residues 27-49 are colored blue for clarity.
| + | |
| - | | + | |
| - | Residues 29-33 line the channel created at the center of the hexamers and stabilize the subunits with the interactions of their side chains and adjacent residues. For example, the "side chain oxygens of Gln29 residues hydrogen bond with the main chain nitrogens of adjacent Gln29 residues, making a circular ring of interactions" and the "valine residues stack upon one another inside the the beta-tube forming a circle of hydrophobic bonds."<ref name=Speir>PMID:7743132</ref> The hydrophobic valine side chain atoms are protected from the interior of the virus by the side chain atoms of the glutamine residue, and they are surrounded by the hydrogen bonding environment of the beta barrels.<ref name=Speir>PMID:7743132</ref>
| + | |
| - | | + | |
| - | In the <scene name='Cowpea_Chlorotic_Mottle_Virus/Interior_of_beta_barrel/1'>interior of beta barrel</scene> we can see these residues filling the channel formed at the center of the capsomere: glutamine 29 (in red), valine 31 (in orange), and valine 33 (in green).
| + | |
| - | | + | |
| - | Pentamer capsomeres, on the other hand, are formed exclusively from the contribution of A subunit chains.
| + | |
| - | <scene name='Cowpea_Chlorotic_Mottle_Virus/Pentamer/1'>Pentamer capsomeres</scene> also contain barrel structures but the amino-terminus arms cluster to create 5-fold symmetry. They have a disordered amino terminus, and residues before Lys42 do not have detectable electron density for the techniques used to render the structure. The positively charged Lys 42 residue is colored in blue as a marker for the arms of the N-terminus.
| + | |
| - | | + | |
| - | Interestingly, although the subunits are chemically identical, hexamer formation predominates in the capsid. In 1962 Caspar and Klug predicted a classical model for a perfect icosahedral structure- one that would have "a sheet of hexamers interspersed with 12 pentamers arranged to form a closed shell with icosahedral symmetry." In this geometrical shape, the number of pentamers is constrained to 12, a structure with greater than 60 faces must be accounted for by an increased number of hexamers. For viruses, a perfect 60-faced icosahedron would severely limit genome packaging.[http://www.nlv.ch/Virologytutorials/Structure.htm]
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | Prior to the characterization of CCMV's structure, no RNA viruses (in plants and insects) were found to rigorously observe this model. Their prediction necessitated that the pentamer and hexamer subunits would from a single chemical structure (realized in CCMV due to its identical A,B, and C gene products). However, the "molecular switch" that determines whether a pentamer or a hexamer would form, was left undefined.
| + | |
| - | It appears that after dimer formation, hexamers predominate in solution. The authors propose that the hexamers then form nucleation sites for particle formation. The basis for the additional hexamer stability comes from the relative number of interactions between molecules.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | <ref name=Speir>PMID:7743132</ref>
| + | |
| - | {{Reflist}}
| + | |