Carbon monoxide dehydrogenase
From Proteopedia
(Difference between revisions)
| (14 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='CODH2-nBIC-Dimer1.pdb' size='450' side='right' scene='Journal:JBIC:13/Cv/5' caption='Carbon monoxide dehydrogenase showing Fe4-S4, Fe2-S2, Fe3-Ni-S4 clusters complex with butylformamide, butyl isocyanate and Fe+3 ion (PDB code [[2yiv]]) '> | |
| - | + | ||
| + | __TOC__ | ||
| - | + | ==Function== | |
| - | + | '''Carbon monoxide dehydrogenase''' (CODH) catalyzes the reversible conversion of CO to CO2. Two classes of CODH were identified: CODH containing 2Fe-Mo-2S-FAD cluster and CODH containing Fe3-Ni-S4 cluster. CODH can exist as a monofunctional enzyme and as a bifunctional enzyme with acetyl-CoA synthase (ACS) (see [[Acetyl-CoA synthase]] and [[Reductive acetyl CoA pathway]]). | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | '''Carbon monoxide dehydrogenase''' (CODH) catalyzes the reversible conversion of CO to CO2. | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==={{nowrap|N-Butylisocyanide Oxidation}} at the {{nowrap|[NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>]-cluster}} of Carbon monoxide dehydrogenase=== | ==={{nowrap|N-Butylisocyanide Oxidation}} at the {{nowrap|[NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>]-cluster}} of Carbon monoxide dehydrogenase=== | ||
Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO<sub>2</sub> as their sole carbon and/or energy source. | Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO<sub>2</sub> as their sole carbon and/or energy source. | ||
| - | + | ||
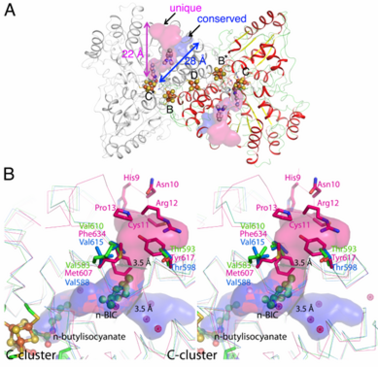
The <scene name='Journal:JBIC:13/Cv/6'>structures of CODHs are homodimers</scene> with ~ 130 kDa containing <scene name='Journal:JBIC:13/Cv/18'>five metal clusters</scene>, called B, B’, C, C’ and D. Each subunit contains the <scene name='Journal:JBIC:13/Cv/19'>active site C-cluster</scene> and cubane-type [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/20'>B-cluster</scene>. Another [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/22'>D-cluster</scene> connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>] (CO + H<sub>2</sub>O ↔ CO<sub>2</sub> + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H<sub>2</sub> and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster. | The <scene name='Journal:JBIC:13/Cv/6'>structures of CODHs are homodimers</scene> with ~ 130 kDa containing <scene name='Journal:JBIC:13/Cv/18'>five metal clusters</scene>, called B, B’, C, C’ and D. Each subunit contains the <scene name='Journal:JBIC:13/Cv/19'>active site C-cluster</scene> and cubane-type [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/20'>B-cluster</scene>. Another [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/22'>D-cluster</scene> connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>] (CO + H<sub>2</sub>O ↔ CO<sub>2</sub> + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H<sub>2</sub> and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster. | ||
| Line 55: | Line 14: | ||
<scene name='Journal:JBIC:13/Cv1/3'>The n-BIC molecule is located in a distance of approximately 8 A from the n-butylisocyanate bound in the active site, measured between nearest atoms and is placed within a hydrophobic channel</scene>, which was shown to be a binding place for Xe atoms in CODH/ACS ([[2z8y]]). Analysis of the CODH-II structure identified the presence of two different channels (see below), in which one is analogous to the channel identified by Xe-soaking in bifunctional CODH/ACS (conserved channel), while the other seems to be specific for monofunctional CODH (unique channel). The conserved channel in CODH-II is similar in position to that in bifunctional CODH, where the channel is extended to reach the A-cluster of ACS. Monofunctional CODHs have smaller side chains like Thr and Val, while Tyr617 and Phe634 block a passage of the unique channel to the protein surface in bifunctional CODH/ACS. Furthermore, the unique channel is completely blocked by residue His9 – Pro13 to prevent diffusion of gaseous substrate/product from the protein in bifunctional CODH/ACS. However, the channel of monofunctional CODH-II is directed towards the solvent, which is in line with its role to allow fast progress and egress of substrates and products from the active site to the outside of the enzyme, which has not been described previously.<ref>DOI 10.1007/s00775-011-0839-y</ref> | <scene name='Journal:JBIC:13/Cv1/3'>The n-BIC molecule is located in a distance of approximately 8 A from the n-butylisocyanate bound in the active site, measured between nearest atoms and is placed within a hydrophobic channel</scene>, which was shown to be a binding place for Xe atoms in CODH/ACS ([[2z8y]]). Analysis of the CODH-II structure identified the presence of two different channels (see below), in which one is analogous to the channel identified by Xe-soaking in bifunctional CODH/ACS (conserved channel), while the other seems to be specific for monofunctional CODH (unique channel). The conserved channel in CODH-II is similar in position to that in bifunctional CODH, where the channel is extended to reach the A-cluster of ACS. Monofunctional CODHs have smaller side chains like Thr and Val, while Tyr617 and Phe634 block a passage of the unique channel to the protein surface in bifunctional CODH/ACS. Furthermore, the unique channel is completely blocked by residue His9 – Pro13 to prevent diffusion of gaseous substrate/product from the protein in bifunctional CODH/ACS. However, the channel of monofunctional CODH-II is directed towards the solvent, which is in line with its role to allow fast progress and egress of substrates and products from the active site to the outside of the enzyme, which has not been described previously.<ref>DOI 10.1007/s00775-011-0839-y</ref> | ||
| - | [[Image:IM.png|left|378px|thumb|(A) Channels in monofunctional CODH-II<sub>''Ch''</sub>. The channel into which the alkyl group of n-BIC is directed, shown in < | + | [[Image:IM.png|left|378px|thumb|(A) Channels in monofunctional CODH-II<sub>''Ch''</sub>. The channel into which the alkyl group of n-BIC is directed, shown in <span style="color:pink;background-color:black;font-weight:bold;">pink color</span>, is unique to monofunctional CODHs. <font color='blue'><b>Blue space-filling model shows the channel found in both mono- and bi-functional CODHs.</b></font> (B) Comparison of channels in mono- (CODH-II<sub>''Ch''</sub> in <span style="color:green;background-color:black;font-weight:bold;">green</span> from this study and CODH from ''Rhodospirillum rubrum'' in <span style="color:cyan;background-color:black;font-weight:bold;">cyan</span> ([[1jqk]])) and bi-functional (CODH from ''Moorella thermoacetica'' in <span style="color:pink;background-color:black;font-weight:bold;">pink</span> from PDB-ID [[2z8y]]) CODHs. <span style="color:pink;background-color:black;font-weight:bold;">Pink-colored dots</span> are Xenon atoms found in CODH from ''Moorella thermoacetica''.]] |
| + | |||
| + | ==3D structures of carbon monoxide dehydrogenase== | ||
| + | [[Carbon monoxide dehydrogenase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
{{Clear}} | {{Clear}} | ||
| - | ==3D structures of carbon monoxide dehydrogenase== | ||
| - | |||
| - | '''CODH monofunctional''' | ||
| - | |||
| - | [[1ffu]] – HpCODH + Fe2S2 + FAD – ''Hydrogenophaga pseudoflava''<br /> | ||
| - | [[1ffv]] - HpCODH + Fe2S2 + molybdenum cofactor + FAD<br /> | ||
| - | [[1jqk]] - CODH + Fe4S4 + Fe + Fe3-Ni-S4 – ''Rhodospirillum rubrum''<br /> | ||
| - | [[1n60]] - OcCODH + Fe2S2 + H2MoO3 + pterin cytosine dinucleotide + FAD – ''Oligotropha carboxidovorans''<br /> | ||
| - | [[1n61]], [[1n62]], [[1n63]], [[1n5w]], [[1zxi]] - OcCODH + Fe2S2 + Cu-Mo cluster + pterin cytosine dinucleotide + FAD<br /> | ||
| - | [[1su6]], [[1su7]], [[1su8]], [[1suf]] - ChCODH + Fe4S4 + Fe2S2 + Fe4-Ni-S5 – ''Carboxydothermus hydrogenoformans''<br /> | ||
| - | [[3b51]], [[3b53]] - ChCODH + Fe4S4 + Fe2S2 + Fe + Fe3-Ni <br /> | ||
| - | [[3b52]] - ChCODH + Fe4S4 + Fe2S2 + Fe + CO2 + Fe3-Ni-S4<br /> | ||
| - | [[3i39]] - ChCODH (mutant) + Fe4S4 + Fe2S2 + Fe + CN + Fe3-Ni-S4<br /> | ||
| - | [[2yiv]] - ChCODH (mutant) + Fe4S4 + Fe2S2 + Fe + butyl-isocyanide + Fe3-Ni-S4<br /> | ||
| - | |||
| - | |||
| - | '''CODH/ACS bifunctional''' | ||
| - | |||
| - | [[1mjg]], [[1oao]], [[2z8y]], [[3i01]] – MtCODH/ACS α+β + Fe4-Ni-S4 – ''Moorella thermoacetica''<br /> | ||
| - | [[3i04]] - MtCODH/ACS α+β + CN + Fe4-Ni-S4<br /> | ||
| - | [[3git]] - MtCODH/ACS α + Fe4S4<br /> | ||
| - | [[3s2x]] - MtCODH/ACS α C terminal + Ni<br /> | ||
| - | [[2h9a]] - ChCODH/ACS γ + Fe4S4<br /> | ||
| - | [[2ycl]] - ChCODH/ACS γ + Fe4S4 + cobalamin | ||
| + | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Jeoung JH, Dobbek H. n-Butyl isocyanide oxidation at the [NiFe(4)S (4)OH ( x )] cluster of CO dehydrogenase. J Biol Inorg Chem. 2011 Sep 9. PMID:21904889 doi:10.1007/s00775-011-0839-y