We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Adrenergic receptor
From Proteopedia
(Difference between revisions)
m |
|||
| (62 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
[[Image:B2ar Image3.png|left|200px|thumb|Crystal Structure of β-2 Adrenergic Receptor, [[2rh1]]]] | [[Image:B2ar Image3.png|left|200px|thumb|Crystal Structure of β-2 Adrenergic Receptor, [[2rh1]]]] | ||
| - | {{STRUCTURE_2rh1| PDB=2rh1 | SIZE=300| SCENE=Beta-2_Adrenergic_Receptor/Opening/1 |right|CAPTION=β-2 Adrenergic Receptor, [[2rh1]] }} | ||
| - | + | <StructureSection load='2r4r' name='main' size='350' side='right' caption='β-2 Adrenergic Receptor (grey) complex with antibody heavy chain (red) and light chain (aqua) (PDB code [[2r4r]]).' scene=''> | |
| + | __TOC__ | ||
| + | ==Function== | ||
| + | The [[Adrenergic receptor|adrenergic receptors]] are metabolic G protein-coupled receptors. They are the targets of catecholamines. The binding of an agonist to them causes a sympathetic response.<br /> | ||
| + | * The '''α-2 adrenergic receptor''' (A2AR) inhibits insulin or glucagons release.<br /> | ||
| + | * The '''β-1 adrenergic receptor''' (B1AR) increases cardiac output and secretion of rennin and ghrelin.<ref>PMID:23435379</ref><br /> | ||
| + | * '''The β-2 adrenergic receptor''' (B2AR) triggers many relaxation reactions. See [[Beta-2 adrenergic receptor]] and [[Beta2 adrenergic receptor-Gs protein complex updated]]<br /> | ||
| + | * The '''β-3 adrenergic receptor''' (B3AR) is active in lipid metabolism.<ref>PMID:9131260</ref><br /> | ||
| + | See also<br /> | ||
| + | * [[G protein-coupled receptor]]<br /> | ||
| + | * [[Beta2 adrenergic receptor-Gs protein complex]]<br /> | ||
| + | * [[Group:SMART:A Physical Model of the β2-Adrenergic Receptor|The Madison West High School 2008 SMART Team's Page on the β-2 adrenergic receptor]]<br /> | ||
| + | * [[UMass Chem 423 Student Projects 2011-1]]<br /> | ||
| + | * [[Neurodevelopmental Disorders]] | ||
| + | * [[Transmembrane (cell surface) receptors]]. | ||
| + | <scene name='44/448705/1/2' target='main'>Click here to see transition from active to inactive conformation of alpha adrenergic receptor</scene> (morph was taken from [http://molmovdb.org/cgi-bin/movie.cgi Gallery of Morphs] of the [http://molmovdb.org Yale Morph Server]). | ||
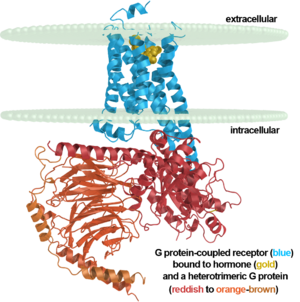
| + | <table width=309' align='right' cellpadding='0'><tr><td rowspan='2'> </td><td bgcolor='#eeeeee'>[[Image:7tm labeled.png|right|300px]]</td></tr><tr><td bgcolor='#eeeeee'><center> | ||
| + | β2 adrenergic receptor binding a hormone analog<br/> and complexed to a heterotrimeric G protein ([[3sn6]]) | ||
| + | </center></td></tr></table> | ||
| + | {{Template:GPCR3sn6}}<br /> | ||
| + | ==Relevance== | ||
| + | [[Alprenolol]] and [[Propranolol]] are B2AR antagonists used in treatment of angina pectoris. [[Timolol]] ia a B2AR antagonist used in treatment of glaucoma. [[Carvedilol]] is A2AR and B2AR antagonist used in treatment of high blood pressure. [[Salbutamol]] (Ventolin) and [[Salmeterol]] are B2AR agonists used for muscle relaxation in asthma attacks. | ||
== 3D Structures of Adrenergic receptor == | == 3D Structures of Adrenergic receptor == | ||
| + | [[Adrenergic receptor 3D structures]] | ||
| - | ''Update November 2011'' | ||
| - | + | This is the first structure of an activated GPCR in a complex with its G protein.<br /> | |
| - | + | {{Template:Robert and Kobilka Nobel Prize}} | |
| - | + | ||
| - | + | ||
| - | ===β-1 adrenergic receptor=== | ||
| - | [[2y01]] – tB1AR fragment (mutant) – turkey<br /> | ||
| - | [[1dep]] – tB1AR peptide - NMR<br /> | ||
| - | [[2y00]], [[2y02]], [[2y03]], [[2y04]], [[2vt4]] - tB1AR fragment (mutant) + agonist<br /> | ||
| - | [[2ycw]], [[2ycx]], [[2ycz]], [[2ycy]] - tB1AR fragment (mutant) + antagonist | ||
| - | ===β-2 adrenergic receptor=== | ||
| - | {{Template:GPCR3sn6}} | ||
| - | See also [[Beta-2 Adrenergic Receptor]]<br /> | ||
| - | [[3pds]] - hB2AR/T4 lysozyme - human<br /> | ||
| - | [[3p0g]] – hB2AR/T4 lysozyme + cameloid antibody fragment<br /> | ||
| - | [[2r4s]],[[2r4r]] - hB2AR + Fab5 complex<br /> | ||
| - | [[2rh1]] - hB2AR/T4 lysozyme (mutant)<br /> | ||
| - | [[3d4s]] - hB2AR/T4 lysozyme (mutant) + cholesterol<br /> | ||
| - | [[3ny8]], [[3ny9]], [[3nya]] – hB2AR + agonist<br /> | ||
| - | [[3sn6]] - human β-2 adrenergic receptor + cameloid antibody fragment bound by guanine nucleotide-binding protein G. '''This is the first structure of an activated GPCR in a complex with its G protein.'''<br /> | ||
| - | See also [[Group:SMART:A Physical Model of the β2-Adrenergic Receptor|The Madison West High School 2008 SMART Team's Page on the β-2 adrenergic receptor]] | ||
| - | {{Template:Robert and Kobilka Nobel Prize}} | ||
| - | ==References | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </StructureSection> | ||
| + | |||
| + | ==References== | ||
<references/> | <references/> | ||
| Line 51: | Line 65: | ||
*[[Membrane proteins]] | *[[Membrane proteins]] | ||
*[[Hormone]] | *[[Hormone]] | ||
| + | *[[Receptor]] | ||
| + | *[[Transmembrane (cell surface) receptors]] | ||
| + | |||
==External Resources== | ==External Resources== | ||
Current revision
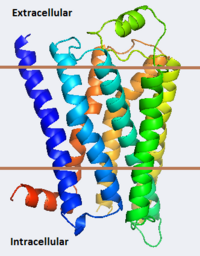
Crystal Structure of β-2 Adrenergic Receptor, 2rh1
| |||||||||||
References
- ↑ Huang J, Chen S, Zhang JJ, Huang XY. Crystal structure of oligomeric beta1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol. 2013 Apr;20(4):419-25. doi: 10.1038/nsmb.2504. Epub 2013 Feb, 24. PMID:23435379 doi:10.1038/nsmb.2504
- ↑ Strosberg AD. Structure and function of the beta 3-adrenergic receptor. Annu Rev Pharmacol Toxicol. 1997;37:421-50. PMID:9131260 doi:10.1146/annurev.pharmtox.37.1.421
- ↑ Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
- ↑ Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007 Nov 23;318(5854):1266-73. Epub 2007 Oct 25. PMID:17962519
- ↑ Ranganathan R. Biochemistry. Signaling across the cell membrane. Science. 2007 Nov 23;318(5854):1253-4. PMID:18033872 doi:10.1126/science.1151656
- ↑ Schwartz TW, Sakmar TP. Structural biology: snapshot of a signalling complex. Nature. 2011 Sep 28;477(7366):540-1. doi: 10.1038/477540a. PMID:21956322 doi:10.1038/477540a
- ↑ Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55. doi: 10.1038/nature10361. PMID:21772288 doi:10.1038/nature10361
- ↑ Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL Jr, Sunahara RK. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011 Sep 28;477(7366):611-5. doi: 10.1038/nature10488. PMID:21956331 doi:10.1038/nature10488
- ↑ Schwartz TW, Sakmar TP. Structural biology: snapshot of a signalling complex. Nature. 2011 Sep 28;477(7366):540-1. doi: 10.1038/477540a. PMID:21956322 doi:10.1038/477540a
See Also
- G protein-coupled receptor
- Beta-2 Adrenergic Receptor topic page
- Nobel Prizes for 3D Molecular Structure
- Highest impact structures of all time
- G proteins
- Rhodopsin
- GTP-binding protein
- Pharmaceutical Drugs
- Membrane proteins
- Hormone
- Receptor
- Transmembrane (cell surface) receptors
External Resources
- Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2007) and the first activated GPCR in complex with its G protein (2011). A detailed description of the laureates' body of work on this class of receptors with images is here.
- The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Alexander Berchansky