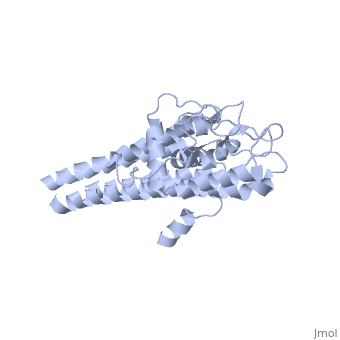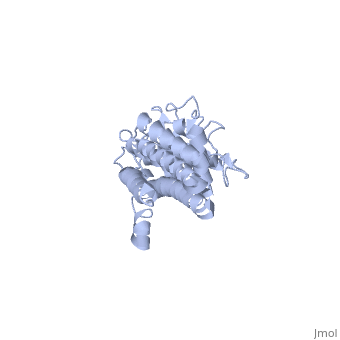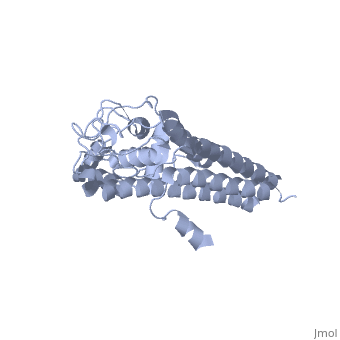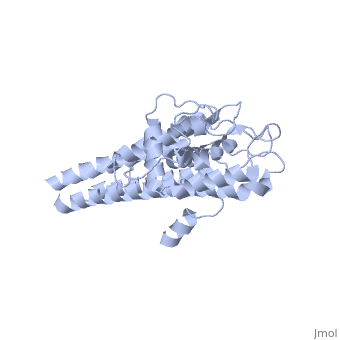
VlsE of Borrelia
From Proteopedia
(Difference between revisions)
(→Variable Domain) |
|||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='1L8W' size='450' side='right' scene='' caption='VlsE (PDB code [[1l8w]])'> | ||
Lyme disease is a multistage, tick-borne infection that is prevalent in the United States, Europe, and Asia. The contributing bacterium that is related to this disease is the spirochete, ''Borrelia burgdorferi''. VlsE is the lipoprotein of the ''Borrelia burgdorferi'' bacteria, which undergoes antigenic variation and plays a major role in the immune response to Lyme disease. A lipoprotein is a structure that contains both proteins and lipids, which allows the protein to integrate with the plasma membrane as a surface protein. It has an effective strategy developed by pathogenic microorganisms to evade the host immune system. The immune system normally reacts to antigens by producing particular antibodies to target that specific antigen. Memory B-cells will retain the proper antigenic counter for the foreign body. However, a variable antigen will frequently change the binding site, so the body will have to constantly look for a new antibody to target. Lyme disease can cause chronic neurologic, cardiovascular, and arthralgic manifestations lasting from months to years. Its persistence is due to the variable properties of the antigen, therefore it is important to understand the structure of vlsE to better counter this disease. | Lyme disease is a multistage, tick-borne infection that is prevalent in the United States, Europe, and Asia. The contributing bacterium that is related to this disease is the spirochete, ''Borrelia burgdorferi''. VlsE is the lipoprotein of the ''Borrelia burgdorferi'' bacteria, which undergoes antigenic variation and plays a major role in the immune response to Lyme disease. A lipoprotein is a structure that contains both proteins and lipids, which allows the protein to integrate with the plasma membrane as a surface protein. It has an effective strategy developed by pathogenic microorganisms to evade the host immune system. The immune system normally reacts to antigens by producing particular antibodies to target that specific antigen. Memory B-cells will retain the proper antigenic counter for the foreign body. However, a variable antigen will frequently change the binding site, so the body will have to constantly look for a new antibody to target. Lyme disease can cause chronic neurologic, cardiovascular, and arthralgic manifestations lasting from months to years. Its persistence is due to the variable properties of the antigen, therefore it is important to understand the structure of vlsE to better counter this disease. | ||
== Structure of VlsE == | == Structure of VlsE == | ||
| - | + | <scene name='VlsE_of_Borrelia/Vlse/1'>VlsE</scene> exists as a <scene name='VlsE_of_Borrelia/Dimer/2'>dimer</scene> molecule in its natural form, comprised of two identical molecules bonded together through weak ionic interactions. The surface area interactions caused by dimerization, along with the variable and invariable <scene name='VlsE_of_Borrelia/Domains/3'>domains</scene>, are central to understanding vlsE. | |
| - | VlsE contains N and C terminus, which correspond to the start of the amino acid sequence to the end, respectively. Unlike other proteins, the N and C terminus regions are neighbored and very flexible. Due to the fact that these regions do not change sequence, they are heavily used for diagnostic tests and vaccination experiments. There is little information on the antigenicity of the '''<span style="color:dimgray">N-terminal</span>''', however, the '''<span style="color:silver">C-terminal</span>''' region is found to be highly immunogenic. Experiments involving treatment with antisera have shown the alpha structure of the C-terminal region associated with the immunogenicity to vary among Lyme disease ''Borrelia'' <ref>Chandra A,Latov N, Wormser GP, Marques AR, Alaedini A. 2011.Epitope Mapping of Antibodies to VlsE Protein of Borrelia burgdorferi in Post-Lyme Disease Syndrome. Clinical Immunology.141(1): 103–110.</ref>. The peptide associated with this immunogenicity is membrane proximal and surface exposed, although crowding of vlsE proteins or bonding to other proteins may block exposure | + | VlsE contains N and C terminus, which correspond to the start of the amino acid sequence to the end, respectively. Unlike other proteins, the N and C terminus regions are neighbored and very flexible. Due to the fact that these regions do not change sequence, they are heavily used for diagnostic tests and vaccination experiments. There is little information on the antigenicity of the '''<span style="color:dimgray">N-terminal</span>''', however, the '''<span style="color:silver">C-terminal</span>''' region is found to be highly immunogenic. Experiments involving treatment with antisera have shown the alpha structure of the C-terminal region associated with the immunogenicity to vary among Lyme disease ''Borrelia'' <ref>Chandra A,Latov N, Wormser GP, Marques AR, Alaedini A. 2011.Epitope Mapping of Antibodies to VlsE Protein of Borrelia burgdorferi in Post-Lyme Disease Syndrome. Clinical Immunology.141(1): 103–110.</ref>. The peptide associated with this immunogenicity is membrane proximal and surface exposed, although crowding of vlsE proteins or bonding to other proteins may block exposure.<ref name="Liang"/> |
''Burgdorferi'' uses antigenic variation to evade the host immune system. It uses genetic recombination as its antigenic variation strategy. The genes coding for vlsE’s variable regions are replaced by replicated vls silent cassette sequences on the B-31 locus, just upstream form the vlsE expression site, that are up to 92% similar to the corresponding vlsE sequences. This gene recombination results in many slightly varied versions of the vlsE antigen. | ''Burgdorferi'' uses antigenic variation to evade the host immune system. It uses genetic recombination as its antigenic variation strategy. The genes coding for vlsE’s variable regions are replaced by replicated vls silent cassette sequences on the B-31 locus, just upstream form the vlsE expression site, that are up to 92% similar to the corresponding vlsE sequences. This gene recombination results in many slightly varied versions of the vlsE antigen. | ||
== Variable Domain == | == Variable Domain == | ||
| - | + | ||
<scene name='VlsE_of_Borrelia/Domains/4'>VlsE</scene> consists of one central <scene name='VlsE_of_Borrelia/Vrs/5'>variable domain</scene> which is comprised of six '''<span style="color:deepskyblue">invariable regions</span>''' (IR<sub>1</sub> to IR<sub>6</sub>), and six '''<span style="color:red">variable regions</span>''' (VR<sub>1</sub> to VR<sub>6</sub>). These variable regions are highly immunogenic; they stimulate an immune response in the body and they serves as a major target of the host immune response. Most of the membrane-distal surface, which covers the α-helix, is made up of coiled forms of the VRs. The placement of these segments on the surface protects the hidden regions from coming into contact with antibodies and therefore serves as the immune evasion from antibody binding. The structure of vlsE indicates that the surface of the protein consists mainly of the variable regions, which go through rapid sequence changes at a high rate during the early stages of an infection. The variable regions on the surface cover the invariable regions of the protein. The exposed variable regions attract the antibodies and turn the immune system away from the invariable regions, leading to an evasion of the immune system. | <scene name='VlsE_of_Borrelia/Domains/4'>VlsE</scene> consists of one central <scene name='VlsE_of_Borrelia/Vrs/5'>variable domain</scene> which is comprised of six '''<span style="color:deepskyblue">invariable regions</span>''' (IR<sub>1</sub> to IR<sub>6</sub>), and six '''<span style="color:red">variable regions</span>''' (VR<sub>1</sub> to VR<sub>6</sub>). These variable regions are highly immunogenic; they stimulate an immune response in the body and they serves as a major target of the host immune response. Most of the membrane-distal surface, which covers the α-helix, is made up of coiled forms of the VRs. The placement of these segments on the surface protects the hidden regions from coming into contact with antibodies and therefore serves as the immune evasion from antibody binding. The structure of vlsE indicates that the surface of the protein consists mainly of the variable regions, which go through rapid sequence changes at a high rate during the early stages of an infection. The variable regions on the surface cover the invariable regions of the protein. The exposed variable regions attract the antibodies and turn the immune system away from the invariable regions, leading to an evasion of the immune system. | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
William Ji, Priya Madan, Maayan Vizana, Garshaw Amidi-Abraham, Alexander Berchansky, Michal Harel