Ciprofloxacin
From Proteopedia
(Difference between revisions)
| (13 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='2662.mol' size='340' side='right' caption='Caption for this structure' scene=''> |
| + | |||
[[Ciprofloxacin]] is a broad-spectrum synthetic fluoroquinolone antibiotic that is effective against both aerobic gram-positive and aerobic gram-negative bacteria<ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref>. | [[Ciprofloxacin]] is a broad-spectrum synthetic fluoroquinolone antibiotic that is effective against both aerobic gram-positive and aerobic gram-negative bacteria<ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref>. | ||
__TOC__ | __TOC__ | ||
| Line 24: | Line 25: | ||
Most anaerobic bacteria exhibit Ciprofloxacin resistance. | Most anaerobic bacteria exhibit Ciprofloxacin resistance. | ||
| - | The effectiveness of Ciprofloxacin against the anthrax-causing bacteria, ''Bacillus anthracis'' has been demonstrated ''in vitro'' and by use of surrogate marker serum levels <ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref><ref>2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.</ref>. Thus, Ciprofloxacin | + | The effectiveness of Ciprofloxacin against the anthrax-causing bacteria, ''Bacillus anthracis'' has been demonstrated ''in vitro'' and by use of surrogate marker serum levels <ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref><ref>2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.</ref>. Thus, Ciprofloxacin, currently, is a Food and Drug Administration (FDA)-approved treatment for patients who have been exposed to anthrax via inhalation<ref>2001. Information on Cipro (Ciprofloxacin Hydrochloride) for Inhalation Anthrax for Consumers: Questions and Answers. Fda.gov. http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130711.htm. Last updated, 2009.</ref>. Likewise, Ciprofloxacin may be used to treat plague (from the bacterium, ''Yersinia pestis'') and tularemia (from the bacterium, ''Francisella tularensis'')<ref>2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.</ref>. Thus, Ciprofloxacin demonstrates usefulness in the field of counter-bioterrorism given its action against bacteria that, potentially, could be implemented in biological warfare. Furthermore, in its extended-release tablet form, Ciprofloxacin targets certain types of urological infectious agents (e.g. those causing epididymitis). The nature of Ciprofloxacin, then, as a powerful, broad-range antibiotic is crucial for broad-range bacterial infection treatment. An understanding of the action of Ciprofloxacin at the molecular level is necessary for an appreciation of the potency of Ciprofloxacin witnessed at the macro level. |
== Historical Information == | == Historical Information == | ||
| - | + | Although the official patented introduction of Ciprofloxacin by Bayer Pharmaceuticals occurred in 1987, some reports indicate that at least two European patents for similar drugs pre-dated the Bayer patent by five years<ref>Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.</ref>. On October 27, 1987, the FDA approved the [[Pharmaceutical Drugs|drug]] for use in the United States for the treatment of certain bacterial infections. The effectiveness of Ciprofloxacin as an antibiotic went unchallenged by all alternative antibiotics<ref>Ciprofloxacin - Activity, Business Aspects/Bayer Pharmaceutical. Encyclopedia.jrank.org. http://encyclopedia.jrank.org/articles/pages/1398940/Ciprofloxacin.html</ref>. Consequently, other pharmaceutical companies were forced to offer their alternative antibiotics at lower costs (compared to the cost of Ciprofloxacin) so as to engage any sort of competition with Bayer Pharmaceuticals. Because of the increasingly common tendency of physicians to prescribe lower-cost medication, Bayer Pharmaceuticals could not expand into the international pharmaceutical market (which, as a whole, was declining) and, consequently, was forced to downsize at the turn of the century. Thus, the competitive effectiveness of Ciprofloxacin did not overcome the competitive pricing of drugs released by other pharmaceutical companies. Faced with the impending expiration of its patent for Ciprofloxacin in the early years of the millennium, Bayer Pharmaceuticals attempted to release variations of Ciprofloxacin. The release of Ciprofloxacin variations such as Pediatric Ciprofloxacin and Once-daily Ciprofloxacin allowed for the extension of the Ciprofloxacin patent. The popularity of Ciprofloxacin rose sharply after September 11, 2001 due to its characteristic targeting of ''Bacillus anthracis'', which was projected as a means of bioterrorism. The prescription of Ciprofloxacin for treatment of bacterial infections continues to this day. | |
== Structure and Administration == | == Structure and Administration == | ||
=== General Quinolone-Fluoroquinolone Structure === | === General Quinolone-Fluoroquinolone Structure === | ||
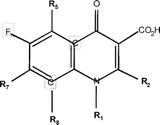
| - | + | Ciprofloxacin is identified as a "quinolone" because of its heterocyclic (with an inner-ring Nitrogen), bicyclic core-containing structure. This structure is characteristic of all quinolones<ref>Siegmund, K., et al. (2005). Molecular details of quinolone-DNA interactions: solution structure of an unusually stable DNA duplex with covalently linked nalidixic acid residues and non-covalent complexes derived from it. ''Nucleic Acids [Research], 33(15)'', 4838-4848.</ref>. Specifically, Ciprofloxacin is characterized as a "fluoroquinolone" since it contains a fluorine atom at the R6 position of its bicyclic core<ref>Peterson, L. (2001). Quinolone-Molecular Structure-Activity Relationships: What We Have Learned About Improving Antimicrobial Activity. ''Clinical Infectious Diseases, 33(3)'', S180-S186.</ref>. All fluoroquinolones contain this R6 fluorine moiety. A general molecular structure for all fluoroquinolones is shown. The R6 fluorine occurs on the left ring of the bicyclic core. | |
[[Image:Flg.jpg]]<ref>Image from: http://cid.oxfordjournals.org/content/33/Supplement_3/S180.full.</ref> | [[Image:Flg.jpg]]<ref>Image from: http://cid.oxfordjournals.org/content/33/Supplement_3/S180.full.</ref> | ||
| Line 42: | Line 43: | ||
=== Administration === | === Administration === | ||
| - | Ciprofloxacin is | + | Usually, Ciprofloxacin is administered either as CIPRO® Oral Suspension (Ciprofloxacin), or as CIPRO® Tablets (Ciprofloxacin hydrochloride)<ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref>. Both administration types are oral. |
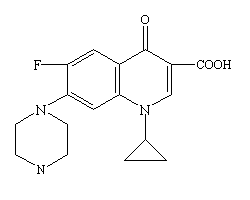
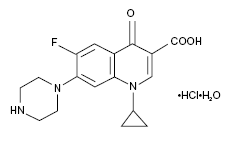
CIPRO® Oral Suspension (Ciprofloxacin) is a 1-cyclopropyl-6-floro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid with empirical formula: C₁₇H₁₈FN₃O₃. Ciprofloxacin has a molecular weight of 331.35 g/mol and occurs as a yellowish, crystalline substance<ref>Molecular weight from Chemexper.com.</ref><ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref>. A simple molecular structure of Ciprofloxacin is shown (base empirical formula). | CIPRO® Oral Suspension (Ciprofloxacin) is a 1-cyclopropyl-6-floro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid with empirical formula: C₁₇H₁₈FN₃O₃. Ciprofloxacin has a molecular weight of 331.35 g/mol and occurs as a yellowish, crystalline substance<ref>Molecular weight from Chemexper.com.</ref><ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref>. A simple molecular structure of Ciprofloxacin is shown (base empirical formula). | ||
| Line 53: | Line 54: | ||
[[Image:ciproHCl.gif]]<ref>Image from: http://www.google.com/imgres?imgurl=http://images.rxlist.com/images/rxlist/ciloxan_s.gif&imgrefurl=http://www.rxlist.com/ciloxan_ophthalmic_ointment-drug.htm&usg=__UqTKseSe8hD85c5RLGIz2_dbAg0=&h=142&w=232&sz=2&hl=en&start=16&zoom=1&tbnid=70Q2WG5hppsQ5M:&tbnh=100&tbnw=164&ei=T010TenMFYH_8Aa6gvDKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C624&um=1&itbs=1&iact=hc&vpx=1064&vpy=399&dur=309&hovh=106&hovw=174&tx=98&ty=76&oei=EU10TcvOCMbdtge5msiLDw&page=2&ndsp=18&ved=1t:429,r:17,s:16&biw=1280&bih=647.</ref> | [[Image:ciproHCl.gif]]<ref>Image from: http://www.google.com/imgres?imgurl=http://images.rxlist.com/images/rxlist/ciloxan_s.gif&imgrefurl=http://www.rxlist.com/ciloxan_ophthalmic_ointment-drug.htm&usg=__UqTKseSe8hD85c5RLGIz2_dbAg0=&h=142&w=232&sz=2&hl=en&start=16&zoom=1&tbnid=70Q2WG5hppsQ5M:&tbnh=100&tbnw=164&ei=T010TenMFYH_8Aa6gvDKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C624&um=1&itbs=1&iact=hc&vpx=1064&vpy=399&dur=309&hovh=106&hovw=174&tx=98&ty=76&oei=EU10TcvOCMbdtge5msiLDw&page=2&ndsp=18&ved=1t:429,r:17,s:16&biw=1280&bih=647.</ref> | ||
| - | Ciprofloxacin | + | Ciprofloxacin also may be administered intravenously and in the form of eye drops or ear drops<ref>Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.</ref>. |
| Line 73: | Line 74: | ||
{{Clear}} | {{Clear}} | ||
=== Topoisomerase IV Target === | === Topoisomerase IV Target === | ||
| - | The structural characterization of the inhibition of DNA replication via inhibition of the action of DNA Topoisomerase IV by Ciprofloxacin is similar to that via inhibition of the action of DNA Gyrase by Ciprofloxacin. An example structure of <scene name='Sandbox_100/Example_topoisomerase_iv/1'>Streptococcus pneumoniae Topoisomerase IV in complex with DNA and attached ligand</scene> is shown (note that this ligand is not Ciprofloxacin, but represents a structure that is analogous to that of Ciprofloxacin). The overall structure of DNA Topoisomerase IV is | + | The structural characterization of the inhibition of DNA replication via inhibition of the action of DNA Topoisomerase IV by Ciprofloxacin is similar to that via inhibition of the action of DNA Gyrase by Ciprofloxacin. An example structure of <scene name='Sandbox_100/Example_topoisomerase_iv/1'>Streptococcus pneumoniae Topoisomerase IV in complex with DNA and attached ligand</scene> is shown (note that this ligand is not Ciprofloxacin, but represents a structure that is analogous to that of Ciprofloxacin). The overall structure of DNA Topoisomerase IV is analogous to that of DNA Gyrase since DNA Topoisomerase IV also appears in an "ironing device" shape with a <scene name='Sandbox_100/Topoisomerase_base_cleft_eg/1'>base cleft forming the active site for interaction with DNA </scene> (in this scene, DNA Topoisomerase IV is light blue, and the dip-like cleft runs the length of the base of the protein; the DNA ligand is black and the example Ciprofloxacin structural analog, as in all scenes under this heading, maintains its atomic color labels). The ligand depicted here intercalates within the DNA structure slightly more aggressively compared to Ciprofloxacin within its DNA Gyrase target (see above), since the DNA structure in this case is <scene name='Sandbox_100/Topo_intercalation/1'>slightly more agitated</scene>(in this scene, DNA is in mesh formation). Yet the concept of obstruction of DNA motility via intercalation applies equivalently in this case and, thus, this model is sufficient for the action of Ciprofloxacin on DNA within DNA Topoisomerase IV. As expected, based on the aforementioned structural similarities, the interactions between the intercalating ligand and the active site of DNA Topoisomerase IV are similar to those witnessed between Ciprofloxacin and DNA Gyrase. The active site of the protein is composed, primarily, of <scene name='Sandbox_100/Active_site_topo_iv_ligand/1'>alpha helices, with polar amino acid residues facing characteristically polar atoms within the structure of the intercalating ligand</scene> (in this scene, alpha helices are purple and polar amino acids on these alpha helices are blue). |
{{Clear}} | {{Clear}} | ||
=== Efflux Pump Interaction === | === Efflux Pump Interaction === | ||
| - | Certain bacteria (''Escherichia coli'', for example) contain a proton motive force-dependent multidrug <scene name='Ciprofloxacin/Cv/1'>efflux pump</scene>, which, as the name suggests, grants the bacteria resistance to certain drugs <ref>Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. ''Journal of Bacteriology, 188(20)'', 7290-7296. </ref>. In ''Escherichia coli'', the efflux system that confers | + | Certain bacteria (''Escherichia coli'', for example) contain a proton motive force-dependent multidrug <scene name='Ciprofloxacin/Cv/1'>efflux pump</scene>, which, as the name suggests, grants the bacteria resistance to certain drugs <ref>Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. ''Journal of Bacteriology, 188(20)'', 7290-7296. </ref>. In ''Escherichia coli'', the efflux system that confers drug resistance is a tripartite transmembrane resistance structure known as "AcrAB-TolC" <ref>Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. ''Molecular Microbiology, 78(2)'', 320-330. </ref>. The drug molecule targeted for excretion is captured by the AcrB subunit (most likely from the periplasm or from the periplasm-intermembrane interface) and is then passed on to the TolC complex for final export. Of course, one could argue that the most important member of the AcrAB-TolC resistance complex is the member that is responsible for the initial attraction of the target compound, the AcrB subunit. Ciprofloxacin may be <scene name='Sandbox_100/Orientation_of_cipro_on_acrb/1'>captured by the AcrB subunit</scene> for removal from the bacterial cell (in this scene, AcrB is in the proposed transmembrane orientation assuming lower cytosolic face and upper exoplasmic face). It has been shown that <scene name='Sandbox_100/Phe_residues/1'> Phe 386 and Phe 388</scene> contribute to the effectiveness of the initial affinity of AcrB for all targets <ref>Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. ''Journal of Bacteriology, 188(20)'', 7290-7296. </ref> (in this scene, both Phe residues are magenta). It also has been shown that, after ligand binding, a proton may bind to an acidic residue in the transmembrane domain, which contains a, as yet, putative network of electrostatically interacting residues, the perturbation of which interacting residues leads to a series of conformational changes that result in drug expulsion. Some residues involved in this chain of events are <scene name='Sandbox_100/Asp_407_408_efflux/1'>Asp 407, Asp 408</scene>, <scene name='Sandbox_100/Lys_940_efflux/1'>Lys 940</scene> and <scene name='Sandbox_100/Thr_178_efflux/1'>Thr 978</scene> (red, purple, green, respectively). The precise mechanism of the action of the AcrB efflux subunit (and of the tripartite AcrAB-TolC in general) is still under scrutiny. |
{{Clear}} | {{Clear}} | ||
== Conclusion == | == Conclusion == | ||
| - | As indicated in the explanation of the interaction between Ciprofloxacin and DNA [[Gyrase]], the precise mechanisms of all Ciprofloxacin interactions and transport systems have not been elaborated completely. However, relevant research, particularly for insight on the precise mechanism for AcrB drug efflux, is underway. Regardless, it is clear that the action of Ciprofloxacin ''in vivo'' is important for the treatment of bacterial infections. The action of Ciprofloxacin on protein function seems to provide | + | As indicated in the explanation of the interaction between Ciprofloxacin and DNA [[Gyrase]], the precise mechanisms of all Ciprofloxacin interactions and transport systems have not been elaborated completely. However, relevant research, particularly for insight on the precise mechanism for AcrB drug efflux, is underway. Regardless, it is clear that the action of Ciprofloxacin ''in vivo'' is important for the treatment of bacterial infections. The action of Ciprofloxacin on protein function seems to provide an avenue of research that could lead to new insights on mechanisms for protein function in general. Thus, Ciprofloxacin is a compound of interest in anticipation of a greater understanding of molecular functions. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ 2001. Information on Cipro (Ciprofloxacin Hydrochloride) for Inhalation Anthrax for Consumers: Questions and Answers. Fda.gov. http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130711.htm. Last updated, 2009.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
- ↑ Ciprofloxacin - Activity, Business Aspects/Bayer Pharmaceutical. Encyclopedia.jrank.org. http://encyclopedia.jrank.org/articles/pages/1398940/Ciprofloxacin.html
- ↑ Siegmund, K., et al. (2005). Molecular details of quinolone-DNA interactions: solution structure of an unusually stable DNA duplex with covalently linked nalidixic acid residues and non-covalent complexes derived from it. Nucleic Acids [Research], 33(15), 4838-4848.
- ↑ Peterson, L. (2001). Quinolone-Molecular Structure-Activity Relationships: What We Have Learned About Improving Antimicrobial Activity. Clinical Infectious Diseases, 33(3), S180-S186.
- ↑ Image from: http://cid.oxfordjournals.org/content/33/Supplement_3/S180.full.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Molecular weight from Chemexper.com.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://textbookofbacteriology.net/themicrobialworld/cipro.gif&imgrefurl=http://textbookofbacteriology.net/themicrobialworld/control.html&usg=__wtzKLHB3NssfnODEB224br5-Bcw=&h=200&w=250&sz=2&hl=en&start=0&zoom=1&tbnid=o7VT7s6FFIUrWM:&tbnh=160&tbnw=199&ei=Hk10TaypBcL58AbyvIjKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=527&vpy=300&dur=1709&hovh=160&hovw=200&tx=155&ty=82&oei=EU10TcvOCMbdtge5msiLDw&page=1&ndsp=16&ved=1t:429,r:7,s:0.
- ↑ Molecular weight from: CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://images.rxlist.com/images/rxlist/ciloxan_s.gif&imgrefurl=http://www.rxlist.com/ciloxan_ophthalmic_ointment-drug.htm&usg=__UqTKseSe8hD85c5RLGIz2_dbAg0=&h=142&w=232&sz=2&hl=en&start=16&zoom=1&tbnid=70Q2WG5hppsQ5M:&tbnh=100&tbnw=164&ei=T010TenMFYH_8Aa6gvDKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C624&um=1&itbs=1&iact=hc&vpx=1064&vpy=399&dur=309&hovh=106&hovw=174&tx=98&ty=76&oei=EU10TcvOCMbdtge5msiLDw&page=2&ndsp=18&ved=1t:429,r:17,s:16&biw=1280&bih=647.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://www.chemdrug.com/databases/SYNTHESIS/SYN/09/09000601a.gif&imgrefurl=http://www.chemdrug.com/databases/8_0_dvpytumicutbciwa.html&usg=__TxiDuzCve6C_crxmcPYTpfW5d4s=&h=555&w=678&sz=6&hl=en&start=0&zoom=1&tbnid=xhquLksJBbMnjM:&tbnh=165&tbnw=201&ei=0Y93TdbGI-yI0QGspa25Bw&prev=/images%3Fq%3Dsynthesis%2Bof%2Bciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=346&vpy=105&dur=63&hovh=203&hovw=248&tx=170&ty=128&oei=0Y93TdbGI-yI0QGspa25Bw&page=1&ndsp=16&ved=1t:429,r:1,s:0
- ↑ Ciprofloxacin Oral - Monograph - Ciprofloxacin Hydrochloride. 2009. Medscape.com. http://www.medscape.com/druginfo/monograph cid=med&drugid=7748&drugname=Ciprofloxacin+Oral&monotype=monograph&secid=8.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
- ↑ Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Molecular Microbiology, 78(2), 320-330.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
Proteopedia Page Contributors and Editors (what is this?)
John Ripollone, John E. Ripollone, Alexander Berchansky, David Canner