We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Matrix metalloproteinase
From Proteopedia
(Difference between revisions)
| (21 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
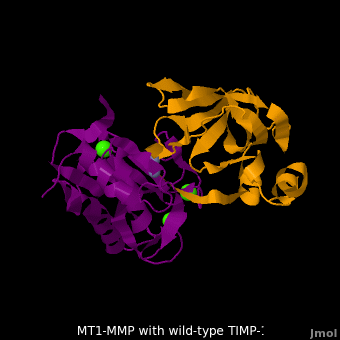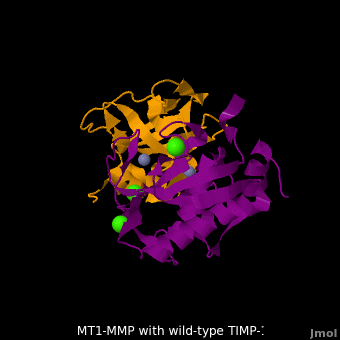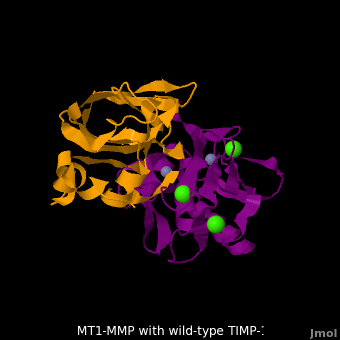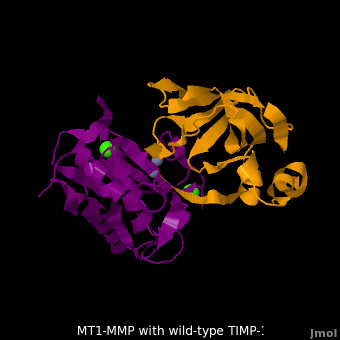
| - | <StructureSection load='M1.pdb' size=' | + | <StructureSection load='M1.pdb' size='350' side='right' scene='MT1-MMP-TIMP-1_complex/Cv2/8' caption='Complex of MMP14 (magenta) and TIMP-1 (orange) with Ca+2 (green) and Zn+2 (grey) ions (PDB code [[3ma2]])'> |
| - | + | ||
| - | + | __TOC__ | |
| - | '''Matrix metalloproteinases''' (MMP) are Zinc-dependent endopeptidases. MMP degrades extracellular matrix proteins | + | == Function == |
| - | [[Matrix metalloproteinases]]<br /> | + | '''Matrix metalloproteinases''' (MMP) are Zinc-dependent endopeptidases. MMP degrades extracellular matrix proteins. They are inhibited by proteases called tissue inhibitors of metalloproteinase (TIMP). The pro-MMP contains a pro-peptide which must be removed to render the MMP active<ref>PMID:10419448</ref>. See details in<br /> |
| - | [[Metalloproteases]]<br /> | + | |
| - | [[MT1-MMP-TIMP-1 complex]]<br />. | + | * [[Matrix metalloproteinases]]<br /> |
| + | * [[Metalloproteases]]<br /> | ||
| + | * [[MT1-MMP-TIMP-1 complex]]<br />. | ||
| + | |||
| + | MMPs are produced by 28 different genes and are classified according to their protein substrates.<br /> | ||
| + | * '''MMP1''' cleaves collagens I, II, III, VII and X.<br /> | ||
| + | * '''MMP2''' cleaves collagen IV and denatured collagen.<br /> | ||
| + | * '''MMP3''' cleaves the core protein of aggrecan and plasminogen activator.<br /> | ||
| + | * '''MMP7''' cleaves proteoglycans, fibronectin, elastin and casein.<br /> | ||
| + | * '''MMP8''' cleaves aggrecan.<br /> | ||
| + | * '''MMP9''' cleaves gelatin. See details in [[Molecular Playground/MMP9]]<br /> | ||
| + | * '''MMP10''' cleaves collagens III, IV, V, fibronectin,gelatin and aggrecan.<br /> | ||
| + | * '''MMP11''' cleaves peptides in human tumors.<br /> | ||
| + | * '''MMP12''' cleaves collagens I and III. See details in [[Matrix Metalloproteinase 12]] <br /> | ||
| + | * '''MMP13''' cleaves collagen II and laminin-5 γ2.<br /> | ||
| + | * '''MMP14''' is a membrane-type MMP which cleaves aggrecan. See details in [[Molecular Playground/MMP14]]<br /> | ||
| + | * '''MMP16''' cleaves collagen III, proteoglycans, fibronectin, gelatin, vitronectin, laminin and α2-macroglobulin.<br /> | ||
| + | * '''MMP20''' cleaves E-cadherin.<br /> | ||
| + | * '''MMP23''' function is unknown.<br /> | ||
| + | * '''MMP adamalysin''' is a snake toxin. See details in [[Atragin]]<br /> | ||
| + | |||
| + | == Relevance == | ||
| + | MMPs have a role in cancer progression<ref>PMID:21087457</ref>. MMP-2 and MMP-9 secretion is elevated in ovarian cancer and are associated with poor prognosis<ref>PMID:19360311</ref>. MMP-8, MMP-9, MMP-13 and MMP-14 have a role in periodontal diseases<ref>PMID:8315570</ref>. | ||
| - | __NOTOC__ | ||
{{Clear}} | {{Clear}} | ||
| Line 16: | Line 37: | ||
account for the entire binding effect between MT1-MMP and TIMP-1. Statistical analysis of the <scene name='MT1-MMP-TIMP-1_complex/Cv2/15'>key hydrogen bond</scene> stabilities in the TIMP-1 T98L mutant reveals that the hydrogen bonds network in mutant form is significantly more stable than that in WT-TIMP-1. Mutations that enhance hydrogen | account for the entire binding effect between MT1-MMP and TIMP-1. Statistical analysis of the <scene name='MT1-MMP-TIMP-1_complex/Cv2/15'>key hydrogen bond</scene> stabilities in the TIMP-1 T98L mutant reveals that the hydrogen bonds network in mutant form is significantly more stable than that in WT-TIMP-1. Mutations that enhance hydrogen | ||
bond stability contribute to the stability of the bound-like, less flexible, conformation of TIMP-1, which eventually results in increasing binding affinity for MT1-MMP. Thus, mutation affected the instrinsic dynamics of the inhibitor rather than its structure, thereby facilitating the interaction <ref name="Grossman">PMID:20545310</ref>. | bond stability contribute to the stability of the bound-like, less flexible, conformation of TIMP-1, which eventually results in increasing binding affinity for MT1-MMP. Thus, mutation affected the instrinsic dynamics of the inhibitor rather than its structure, thereby facilitating the interaction <ref name="Grossman">PMID:20545310</ref>. | ||
| - | </StructureSection> | ||
| - | __NOTOC__ | ||
==3D structures of matrix metalloproteinase== | ==3D structures of matrix metalloproteinase== | ||
| + | [[Matrix metalloproteinase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==References== | ==References== | ||
Current revision
| |||||||||||
References
- ↑ Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999 Jul 30;274(31):21491-4. PMID:10419448
- ↑ Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011 Jan;278(1):16-27. doi: 10.1111/j.1742-4658.2010.07919.x. Epub 2010, Nov 19. PMID:21087457 doi:http://dx.doi.org/10.1111/j.1742-4658.2010.07919.x
- ↑ Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009 May;21(5):1323-33. PMID:19360311
- ↑ Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993 May;64(5 Suppl):474-84. PMID:8315570 doi:http://dx.doi.org/10.1902/jop.1993.64.5s.474
- ↑ Grossman M, Tworowski D, Dym O, Lee MH, Levy Y, Murphy G, Sagi I. Intrinsic protein flexibility of endogenous protease inhibitor TIMP-1 controls its binding interface and effects its function. Biochemistry. 2010 Jun 14. PMID:20545310 doi:10.1021/bi902141x