Sandbox Reserved 765
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 41: | Line 41: | ||
All of the beta sheets within each monomer run anti-parallel with one another. Helices are divided into two categories: alpha helices and 3/10 helices. In the structure there are eleven alpha helices and | All of the beta sheets within each monomer run anti-parallel with one another. Helices are divided into two categories: alpha helices and 3/10 helices. In the structure there are eleven alpha helices and | ||
| - | there are six 3/10 helices. The image to the right displays the sequence of chorismate synthase. We can examine the two structures, <scene name='56/564041/Unbound_chorismate_synthase/1'>unbound</scene> chorismate synthase and with mycobacterium tuberculosis <scene name='56/564041/Bound_chorismate_synthase/1'>bound</scene> and FMN bound. The <scene name='56/564041/N-c_terminal/1'>N and C terminus</scene> are both present within each monomer and goes from blue to red. | + | there are six 3/10 helices. The image to the right displays the sequence of chorismate synthase. We can examine the two structures, <scene name='56/564041/Unbound_chorismate_synthase/1'>unbound</scene> chorismate synthase and with ''mycobacterium tuberculosis'' <scene name='56/564041/Bound_chorismate_synthase/1'>bound</scene> and FMN bound. The <scene name='56/564041/N-c_terminal/1'>N and C terminus</scene> are both present within each monomer and goes from blue to red. |
==Mechanism== | ==Mechanism== | ||
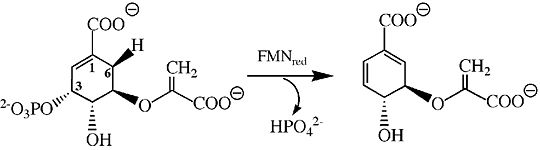
| - | Below is the proposed mechanism for the enzyme chorismate synthase. Since chorismate synthase is part of the lyase family, there will be an elimination reaction with a newly formed double bond. A very important cofactor is needed, Flavin Mononucleotide (FMN), when it is reduced the reaction is able to take place. The predicted model is that <scene name='56/564041/His_106/2'>HIS 106</scene> protonates the monoanionic reduced FMN and then <scene name='56/564041/His_17/2'>HIS 17</scene> protonates the leaving inorganic phosphate group of the substrate <ref>PMID:14668332</ref>. This is consider to be the <scene name='56/564041/Active_site/3'>active site</scene>, where all of the catalytic events partake. Based on current studies, FMN comes in and is protonated immediately by HIS 106 making FMNH2 and leaving. His 17 then protonates the leaving phosphate group to allow for the elimination to be completed <ref>PMID:14668332</ref>. To finish off the reaction, the electrons are shifted around to make a double bond that is necessary for the synthesis of aromatic amino acids. The aromatic amino acids that are form are tyrosine, tyrptophan, and phenylalanine; because of the double bond being formed within the six-membered ring. | + | Below is the proposed mechanism for the enzyme chorismate synthase. Since chorismate synthase is part of the lyase family, there will be an elimination reaction with a newly formed double bond. A very important cofactor is needed, Flavin Mononucleotide (FMN), when it is reduced the reaction is able to take place <ref>PMID:9951731</ref>. The predicted model is that <scene name='56/564041/His_106/2'>HIS 106</scene> protonates the monoanionic reduced FMN and then <scene name='56/564041/His_17/2'>HIS 17</scene> protonates the leaving inorganic phosphate group of the substrate <ref>PMID:14668332</ref>. This is consider to be the <scene name='56/564041/Active_site/3'>active site</scene>, where all of the catalytic events partake. Based on current studies, FMN comes in and is protonated immediately by HIS 106 making FMNH2 and leaving. His 17 then protonates the leaving phosphate group to allow for the elimination to be completed <ref>PMID:14668332</ref>. To finish off the reaction, the electrons are shifted around to make a double bond that is necessary for the synthesis of aromatic amino acids. The aromatic amino acids that are form are tyrosine, tyrptophan, and phenylalanine; because of the double bond being formed within the six-membered ring. |
[[Image:Chorismate Synthase Mechanism.jpg|thumb|540px|Figure 3. The main mechanism of chorismate synthase involving HIS 17.]] | [[Image:Chorismate Synthase Mechanism.jpg|thumb|540px|Figure 3. The main mechanism of chorismate synthase involving HIS 17.]] | ||
If we examine the image above we can see that FMN gets reduced and the phosphate group that is on carbon number 3 within the cyclic structure is removed and replaced by a double bond between carbon 2 and 3. | If we examine the image above we can see that FMN gets reduced and the phosphate group that is on carbon number 3 within the cyclic structure is removed and replaced by a double bond between carbon 2 and 3. | ||
[[Image:HIS 17 and HIS 106.png|260px|left|thumb|This is a representation of the two essential amino acids sites, HIS 17 and HIS 106.]] | [[Image:HIS 17 and HIS 106.png|260px|left|thumb|This is a representation of the two essential amino acids sites, HIS 17 and HIS 106.]] | ||
| - | The image to the left shows the two essential amino acid sites that are necessary for the conversion of 5-enolpyruvylshikimate-3-phosphate into chorismate <ref>PMID:14668332</ref>. | + | The image to the left shows the two essential amino acid sites that are necessary for the conversion of 5-enolpyruvylshikimate-3-phosphate into chorismate <ref>PMID:14668332</ref>. It is required that the two active site be within 3Å in distance for the reaction to take place. This is why we know that HIS 106 is required for the reaction but not sure as to what role it plays exactly. |
| + | |||
| + | ===Inhibition=== | ||
| + | Chorismate synthase can be inhibited by many molecules which is effective for antimicrobial drugs because this process only takes place in microorganisms and plants. One common inhibition is through (6R)-6-Fluoro-5-enolpyruvylshikimate-3-phosphate <ref>PMID:10956653</ref>. This molecule is able to convert 5-enolpyruvylshikimate-3-phosphate into chorismate but stays attached to chorismate preventing any functionality<ref>PMID:10956653</ref>. | ||
==Implications== | ==Implications== | ||
| - | The implications with studying choristmate synthase revolves around the fact that it isn not present in humans but is essential for bacteria, plants and parasites. This pathway gives rise to many potential antimicrobial drugs which decreases possible negative impacts of drugs in humans <ref>PMID:4550759</ref>. | + | Enzymes present in the shikimate pathway are important in microorganism survival. The enzymes of the shikimate pathway are a great source for antimicrobial agents, herbicides, inhibitors and drugs. The implications with studying choristmate synthase revolves around the fact that it isn not present in humans but is essential for bacteria, plants and parasites. This pathway gives rise to many potential antimicrobial drugs which decreases possible negative impacts of drugs in humans <ref>PMID:4550759</ref>. One major disease is ''mycobacterium tuberculosis'', that can be stopped using chemotherapy due to the use of inhibitors that can prevent chorismate to be produced and effect the human body. |
| - | ==References== | ||
| - | {{reflist}} | ||
| + | </StructureSection> | ||
| + | ==References== | ||
| - | + | {{reflist}} | |
Current revision
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Chorismate Synthase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Crystal structure of Chorismate synthase complexed with oxidized FMN and EPSP." RSCB Protein Data Bank. RCSB. Web. 30 Nov. 2013. http://www.rcsb.org/pdb/explore/explore.do?structureId=1qxo.
- ↑ Marchenko LF, Arsen'eva EN, Ignatiuk TE, Semenova EI, Sapelkina LV. [Hydroxyproline and somatotropic hormone in children of mothers with diabetes mellitus]. Pediatriia. 1987;(7):11-3. PMID:3670998
- ↑ Arcuri HA, Palma MS. Understanding the structure, activity and inhibition of chorismate synthase from Mycobacterium tuberculosis. Curr Med Chem. 2011;18(9):1311-7. PMID:21366532
- ↑ Fitzpatrick TB, Killer P, Thomas RM, Jelesarov I, Amrhein N, Macheroux P. Chorismate synthase from the hyperthermophile Thermotoga maritima combines thermostability and increased rigidity with catalytic and spectral properties similar to mesophilic counterparts. J Biol Chem. 2001 May 25;276(21):18052-9. Epub 2001 Mar 9. PMID:11279147 doi:http://dx.doi.org/10.1074/jbc.M100867200
- ↑ Macheroux P, Schmid J, Amrhein N, Schaller A. A unique reaction in a common pathway: mechanism and function of chorismate synthase in the shikimate pathway. Planta. 1999 Jan;207(3):325-34. PMID:9951731
- ↑ Kitzing K, Auweter S, Amrhein N, Macheroux P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J Biol Chem. 2004 Mar 5;279(10):9451-61. Epub 2003 Dec 10. PMID:14668332 doi:http://dx.doi.org/10.1074/jbc.M312471200
- ↑ Kitzing K, Auweter S, Amrhein N, Macheroux P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J Biol Chem. 2004 Mar 5;279(10):9451-61. Epub 2003 Dec 10. PMID:14668332 doi:http://dx.doi.org/10.1074/jbc.M312471200
- ↑ Kitzing K, Auweter S, Amrhein N, Macheroux P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J Biol Chem. 2004 Mar 5;279(10):9451-61. Epub 2003 Dec 10. PMID:14668332 doi:http://dx.doi.org/10.1074/jbc.M312471200
- ↑ Osborne A, Thorneley RN, Abell C, Bornemann S. Studies with substrate and cofactor analogues provide evidence for a radical mechanism in the chorismate synthase reaction. J Biol Chem. 2000 Nov 17;275(46):35825-30. PMID:10956653 doi:http://dx.doi.org/10.1074/jbc.M005796200
- ↑ Osborne A, Thorneley RN, Abell C, Bornemann S. Studies with substrate and cofactor analogues provide evidence for a radical mechanism in the chorismate synthase reaction. J Biol Chem. 2000 Nov 17;275(46):35825-30. PMID:10956653 doi:http://dx.doi.org/10.1074/jbc.M005796200
- ↑ Floss HG, Onderka DK, Carroll M. Stereochemistry of the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J Biol Chem. 1972 Feb 10;247(3):736-44. PMID:4550759