We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 930
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_510042_1}} | {{Sandbox_Reserved_510042_1}} | ||
| - | =Scallop myosin head in | + | =Scallop myosin head in the detached state= |
| Line 27: | Line 27: | ||
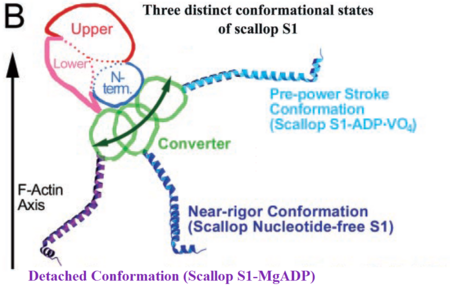
• S1 nucleotide-free state corresponding to the near-rigor conformation of myosin [http://www.pdb.org/pdb/explore/explore.do?structureId=1DFK 1DFK] | • S1 nucleotide-free state corresponding to the near-rigor conformation of myosin [http://www.pdb.org/pdb/explore/explore.do?structureId=1DFK 1DFK] | ||
| - | • S1 Mg-ADP • | + | • S1 Mg-ADP • BeFx state corresponding to the pre-power stroke conformation [http://www.pdb.org/pdb/explore/explore.do?structureId=1kk8 1KK8] |
• S1 Mg-ADP state corresponding to the myosin detached state [http://www.rcsb.org/pdb/explore/explore.do?structureId=1b7t 1B7T] | • S1 Mg-ADP state corresponding to the myosin detached state [http://www.rcsb.org/pdb/explore/explore.do?structureId=1b7t 1B7T] | ||
| - | Comparing the available crystal structures of different myosin S1 units enable us to understand the conformational changes within the motor domain during the contractile cycle. Here mainly the structure and function of the MD in the S1 Mg-ADP | + | Comparing the available crystal structures of different myosin S1 units enable us to understand the conformational changes within the motor domain during the contractile cycle. Here mainly the structure and function of the MD in the S1 Mg-ADP detached state will be discussed. |
| Line 53: | Line 53: | ||
==Nucleotide binding pocket: ADP + Mg<sup>2+</sup>== | ==Nucleotide binding pocket: ADP + Mg<sup>2+</sup>== | ||
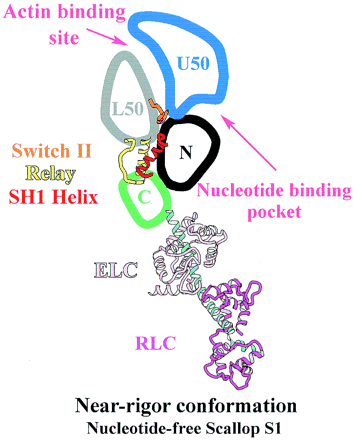
| - | The nucleotide | + | The nucleotide binding pocket is located at the interface of the 50 kDa upper subdomain and the N-terminal subdomain <ref name="risal2004"> PMID: 15184651</ref>, which is opposite to a deep cleft that bisects the actin-binding domain (Fig. 3). The nucleotide binding pocket and actin binding domain contained a complex arrangement of secondary structure elements mainly around the parallel 7-stranded <scene name='57/579700/Strands/1'> β-sheet</scene>. Loops extending from the β-strands interact with the adenine nucleotide. |
| - | <scene name='57/579700/Adp/5'>ADP</scene> forms hydrogen bonds with the amino acid side chains | + | <scene name='57/579700/Adp/5'>ADP</scene> forms hydrogen bonds with the amino acid side chains in the binding pocket. <scene name='57/579700/Mg/5'>Mg2+</scene> coordinates with residues Thr183, Ser 241 of the N-terminal subdomain as well as O1B and O3B from ADP and three water molecules. The hydrogen bonds between ADP and the amino acid residues together with the interactions of Mg2+ keep ADP in the nucleotide-binding pocket. |
In the contractile cycle ATP binding causes a conformational change, which detaches the myosin S1 unit from actin. Then the active site closes, and ATP is hydrolysed to Pi and ADP, leading to the subsequent reattachment of the S1 with the actin. The conformational changes of the acting-binding pocket and the opening and closing of the nucleotide-binding pocket cause the strong and weak acting binding states of myosin, allowing muscle contraction <ref name="houdusse2000"> PMID: 11016966</ref>. | In the contractile cycle ATP binding causes a conformational change, which detaches the myosin S1 unit from actin. Then the active site closes, and ATP is hydrolysed to Pi and ADP, leading to the subsequent reattachment of the S1 with the actin. The conformational changes of the acting-binding pocket and the opening and closing of the nucleotide-binding pocket cause the strong and weak acting binding states of myosin, allowing muscle contraction <ref name="houdusse2000"> PMID: 11016966</ref>. | ||
| Line 73: | Line 73: | ||
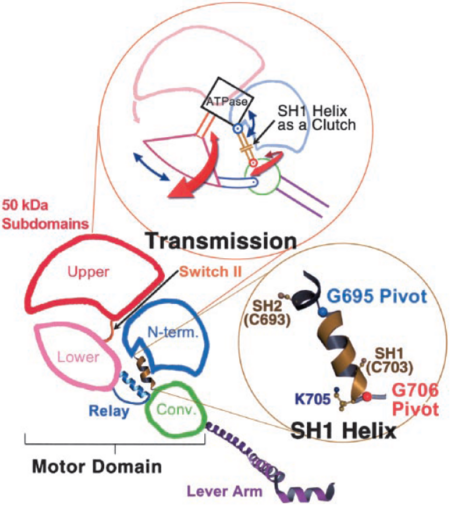
Taking part in the organization of the different conformations of the contractile cycle is also the so called switch I, which is a second catalytic loop of the nucleotide-binding pocket. <ref name="houdusse2000"> PMID: 11016966</ref> Switch II forms a specific salt bridge and hydrogen bond interactions with switch I that stabilize the pre-power stroke state <ref name="himmel2002"> PMID: 12297624</ref>. | Taking part in the organization of the different conformations of the contractile cycle is also the so called switch I, which is a second catalytic loop of the nucleotide-binding pocket. <ref name="houdusse2000"> PMID: 11016966</ref> Switch II forms a specific salt bridge and hydrogen bond interactions with switch I that stabilize the pre-power stroke state <ref name="himmel2002"> PMID: 12297624</ref>. | ||
| - | In the | + | In the detached conformation of the MD, switch II interacts with the nucleotide-binding pocket and forms the stabilizing hydrogen bond interactions and a salt bridge with switch I <ref name="himmel2002"> PMID: 12297624</ref><ref name="risal2004"> PMID: 15184651</ref>. Rotation of the 50-kDa upper subdomain away from the N-terminal subdomain pulls switches I and II apart breaking the protein- nucleotide interactions between switch I and ADP, as well as changing the conformation of switch II. These changes result in closing of the actin-binding site and opening of the nucleotide-binding pocket, leading to MgADP release. At the same time SH1 helix is unwound and the lever arm is able to change its position enabling sliding of myosin through the actin filament <ref name="himmel2002"> PMID: 12297624</ref>. |
Current revision
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Contents |
Scallop myosin head in the detached state
Introduction
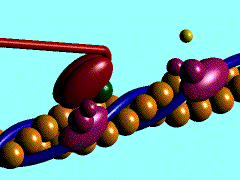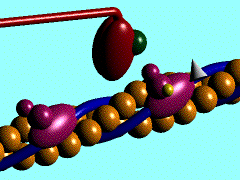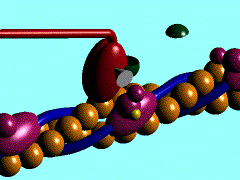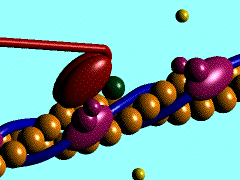
Figure 1. The movement of myosin motor domain on actin filament.[1]
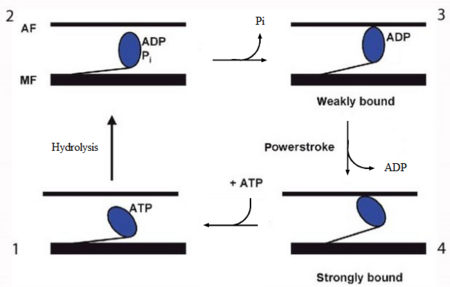
Figure 2. The contractile cycle of the myosin head [2].
In the striated muscle the actin and myosin proteins form ordered basic units called sarcomeres. Muscle contraction is achieved by the mechanical sliding of myosin filament (thick filament) along the actin filament (thin filament), Fig. 1. The major constituent of the myosin filament is myosin, a motor protein responsible for converting chemical energy to mechanical movement. In the presence of Ca2+ and Mg2+ myosin is able to cyclically bind ATP and hydrolyse it to ADP + Pi , thus triggering myosin-actin detachment, reattachment and power stroke, the so called contractile cycle (Fig.2)[2].
.
Introduction of the Myosin head S1
| |||||||||||
References
- ↑ San Diego State University College of Sciences, link
- ↑ 2.0 2.1 Krans, J. 2010. The Sliding Filament Theory of Muscle Contraction. Nature Education 3(9):66.
- ↑ Rayment I, Holden HM. The three-dimensional structure of a molecular motor. Trends Biochem Sci. 1994 Mar;19(3):129-34. PMID:8203020
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Houdusse A, Kalabokis VN, Himmel D, Szent-Gyorgyi AG, Cohen C. Atomic structure of scallop myosin subfragment S1 complexed with MgADP: a novel conformation of the myosin head. Cell. 1999 May 14;97(4):459-70. PMID:10338210
- ↑ 6.0 6.1 6.2 6.3 Risal D, Gourinath S, Himmel DM, Szent-Gyorgyi AG, Cohen C. Myosin subfragment 1 structures reveal a partially bound nucleotide and a complex salt bridge that helps couple nucleotide and actin binding. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8930-5. Epub 2004 Jun 7. PMID:15184651 doi:10.1073/pnas.0403002101
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Himmel DM, Gourinath S, Reshetnikova L, Shen Y, Szent-Gyorgyi AG, Cohen C. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12645-50. Epub 2002 Sep 24. PMID:12297624 doi:10.1073/pnas.202476799