Krebs cycle step 3
From Proteopedia
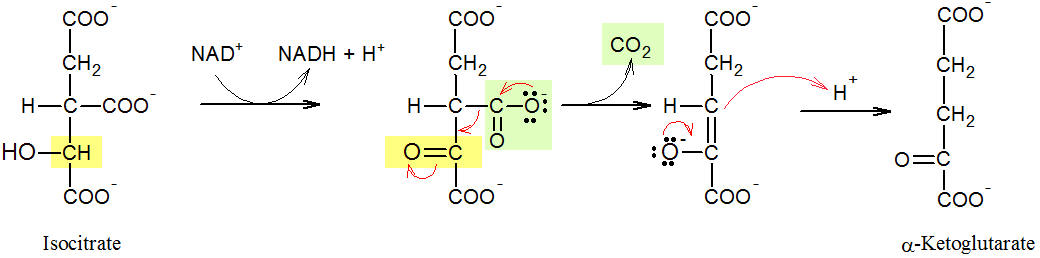
(New page: <h2>Step three of the Krebs Cycle: Isocitrate Dehydrogenase</h2> Image:isocitrate_3.jpg <p>Figure: Formation of α-ketoglutarate</p> <p>Like the second step of the Krebs cycle, ...) |
|||
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
| - | <h2>Step three of the Krebs Cycle: Isocitrate | + | <h2>Step three of the Krebs Cycle: [[Isocitrate dehydrogenase]]</h2> |
[[Image:isocitrate_3.jpg]] | [[Image:isocitrate_3.jpg]] | ||
<p>Figure: Formation of α-ketoglutarate</p> | <p>Figure: Formation of α-ketoglutarate</p> | ||
| - | <p>Like the second step of the Krebs cycle, the third reaction (see Figure) is a two-step reaction sequence. In the first step, | + | <p>Like the second step of the [[Citric Acid Cycle|Krebs cycle]], the third reaction (see Figure) is a two-step reaction sequence. In the first step, |
the secondary OH group of isocitrate (highlighted in yellow) is oxidised by the coenzyme NAD<sup>+</sup>, and a ketone is formed. At this point it becomes apparent why citrate was previously isomerised to isocitrate: Without | the secondary OH group of isocitrate (highlighted in yellow) is oxidised by the coenzyme NAD<sup>+</sup>, and a ketone is formed. At this point it becomes apparent why citrate was previously isomerised to isocitrate: Without | ||
this step, the oxidation would not have taken place, since tertiary alcohols can not be oxidised. In the second step of the | this step, the oxidation would not have taken place, since tertiary alcohols can not be oxidised. In the second step of the | ||
reaction, the intermediate product is decarboxylated and thus CO<sub>2</sub> (in green) and | reaction, the intermediate product is decarboxylated and thus CO<sub>2</sub> (in green) and | ||
α-ketoglutarate, the salt of α-ketoglutaric acid, are released. With this step, the carbon chain is shortened for the first time because α-ketoglutarate has a C5 body. </p> | α-ketoglutarate, the salt of α-ketoglutaric acid, are released. With this step, the carbon chain is shortened for the first time because α-ketoglutarate has a C5 body. </p> | ||
Current revision
Step three of the Krebs Cycle: Isocitrate dehydrogenase
Figure: Formation of α-ketoglutarate
Like the second step of the Krebs cycle, the third reaction (see Figure) is a two-step reaction sequence. In the first step, the secondary OH group of isocitrate (highlighted in yellow) is oxidised by the coenzyme NAD+, and a ketone is formed. At this point it becomes apparent why citrate was previously isomerised to isocitrate: Without this step, the oxidation would not have taken place, since tertiary alcohols can not be oxidised. In the second step of the reaction, the intermediate product is decarboxylated and thus CO2 (in green) and α-ketoglutarate, the salt of α-ketoglutaric acid, are released. With this step, the carbon chain is shortened for the first time because α-ketoglutarate has a C5 body.