Sandbox Reserved 963
From Proteopedia
(Difference between revisions)
| (70 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | ==Anti-amyloid-beta Fab WO2 (Form A, | + | ==Anti-amyloid-beta Fab WO2 (Form A, P2<sub>1</sub>2<sub>1</sub>2<sub>1</sub>)== |
<StructureSection load='3bkm' size='340' side='right' caption='[[3bkm]], [[Resolution|resolution]] 1.60Å' scene=''> | <StructureSection load='3bkm' size='340' side='right' caption='[[3bkm]], [[Resolution|resolution]] 1.60Å' scene=''> | ||
'''Alzheimer's disease (AD)''', is the most common form of dementia. It is a chronic neurodegenerative disease that usually develops slowly and get worse over time, becoming severe enough to interfere with daily tasks. The most common early symptom of Alzheimer's disease is the difficulty in remembering recently learned information. As the patient with Alzheimer’s disease ages, symptoms such as speaking problems, language problems, mood swings, disorientation, behavioural issues, and loss of motivation, can appear. | '''Alzheimer's disease (AD)''', is the most common form of dementia. It is a chronic neurodegenerative disease that usually develops slowly and get worse over time, becoming severe enough to interfere with daily tasks. The most common early symptom of Alzheimer's disease is the difficulty in remembering recently learned information. As the patient with Alzheimer’s disease ages, symptoms such as speaking problems, language problems, mood swings, disorientation, behavioural issues, and loss of motivation, can appear. | ||
| Line 7: | Line 7: | ||
However, many groups of researchers are seeking a solution to this problem and most of them are currently focused on the activity of a small peptide called '''[http://en.wikipedia.org/wiki/Amyloid_beta Amyloid β (Aβ)]'''. | However, many groups of researchers are seeking a solution to this problem and most of them are currently focused on the activity of a small peptide called '''[http://en.wikipedia.org/wiki/Amyloid_beta Amyloid β (Aβ)]'''. | ||
| - | One of the hypothetical causes of this disease is depicted as the presence of the amyloid plaques found in the brains of Alzheimer patients | + | One of the hypothetical causes of this disease is depicted as the presence of the amyloid plaques (which are composed of Aβ) found in the brains of Alzheimer patients. |
These are peptides of 36–43 amino acids, obtained via proteolysis of '''[http://en.wikipedia.org/wiki/Amyloid_precursor_protein Amyloid Precursor Protein (APP)]''' by β and γ secretases. | These are peptides of 36–43 amino acids, obtained via proteolysis of '''[http://en.wikipedia.org/wiki/Amyloid_precursor_protein Amyloid Precursor Protein (APP)]''' by β and γ secretases. | ||
| - | An immunologic approach to the disease is made. Researchers have developped a monoclonal antibody, WO2 which can bind specifically to the Aβ’s epitope, thus leading the complex to be phagocyted. | + | An immunologic approach to the disease is made. Researchers have developped a monoclonal antibody, '''WO2''', which can bind specifically to the Aβ’s epitope, thus leading the complex to be phagocyted. |
== Structural highlights == | == Structural highlights == | ||
The three-dimensional structure of WO2 was obtained thanks to X-ray crystallography. | The three-dimensional structure of WO2 was obtained thanks to X-ray crystallography. | ||
| - | The structure of WO2 that is described here is that of WO2 murine anti-Aβ monoclonal Fab in the space group | + | The structure of WO2 that is described here is that of '''WO2 murine anti-Aβ monoclonal Fab (antigen binding fragment) in the space group P2<sub>1</sub>2<sub>1</sub>2<sub>1</sub> (Form A)'''. There's also another form available for the WO2 antibody, the Form B, but it is not mentioned here because it is crystallized in the space group P2<sub>1</sub>. Thus, in what follows, the different features correspond to the Form A. |
| - | This structure appears to be that of a typical immunoglobulin (Ig) Fab heavy-chain/light-chain heterodimer. The heavy chain is made up of 252 amino acids while the light chain is made up of 224 amino acids. | + | |
| + | This structure appears to be that of a typical immunoglobulin (Ig) Fab heavy-chain/light-chain heterodimer weighting 52362 Da<ref>http://www.rcsb.org/pdb/explore/explore.do?structureId=3bkm</ref>. The heavy chain is made up of 252 amino acids while the light chain is made up of 224 amino acids.<ref>http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?uid=63842</ref> | ||
| + | |||
| + | |||
| + | |||
| + | WO2 contains several helices and sheets<ref>http://oca.weizmann.ac.il/oca-bin/csu?PDB_ID=3bkm#ref1</ref> : | ||
| + | |||
| + | *The structure contains <scene name='60/604482/My_first_scene/41'>9 α-helices</scene> : | ||
| + | ** <scene name='60/604482/My_first_scene/20'>Helix 1</scene> : chain L; Right-handed 310 | ||
| + | ** <scene name='60/604482/My_first_scene/21'>Helix 2</scene> : chain L; Right-handed alpha | ||
| + | ** <scene name='60/604482/My_first_scene/22'>Helix 3</scene> : chain L; Right-handed alpha | ||
| + | ** <scene name='60/604482/My_first_scene/23'>Helix 4</scene> : chain H; Right-handed alpha | ||
| + | ** <scene name='60/604482/My_first_scene/24'>Helix 5</scene> : chain H; Right-handed 310 | ||
| + | ** <scene name='60/604482/My_first_scene/25'>Helix 6</scene> : chain H; Right-handed 310 | ||
| + | ** <scene name='60/604482/My_first_scene/26'>Helix 7</scene> : chain H; Right-handed 310 | ||
| + | ** <scene name='60/604482/My_first_scene/27'>Helix 8</scene> : chain H; Right-handed 310 | ||
| + | ** <scene name='60/604482/My_first_scene/28'>Helix 9</scene> : chain H; Right-handed 310 | ||
| + | |||
| + | *The structure also contains <scene name='60/604482/My_first_scene/29'>11 β-sheets</scene> : | ||
| + | ** <scene name='60/604482/My_first_scene/30'>A sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/31'>B sheet</scene> : 6 strands | ||
| + | ** <scene name='60/604482/My_first_scene/32'>C sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/33'>D sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/34'>E sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/35'>F sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/36'>G sheet</scene> : 6 strands | ||
| + | ** <scene name='60/604482/My_first_scene/37'>H sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/38'>I sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/39'>J sheet</scene> : 4 strands | ||
| + | ** <scene name='60/604482/My_first_scene/40'>K sheet</scene> : 3 strands | ||
| + | |||
| + | |||
| + | Four disulphides bonds are present in the WO2 structure : | ||
| + | |||
| + | * Two in the L chain<ref>http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3bkm&template=protein.html&o=DISULPHIDES&l=1&s=1&c=10&chain=L</ref> : <scene name='60/604482/My_first_scene/42'>between Cys23 and Cys88</scene>, and <scene name='60/604482/My_first_scene/43'>between Cys133 and Cys193</scene> | ||
| + | * Two in the H chain<ref>http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3bkm&template=protein.html&o=DISULPHIDES&l=2&s=1&c=9&chain=H</ref> : <scene name='60/604482/My_first_scene/44'>between Cys22 and Cys92</scene>, and <scene name='60/604482/My_first_scene/45'>between Cys140 and Cys195</scene> | ||
| + | |||
| + | |||
In Form A, we notice an elbow angle of 192°. | In Form A, we notice an elbow angle of 192°. | ||
| - | Constant (C) and variable (V) domains of both light (L) and heavy (H) chains include the following residues : VL(1-107), CL(108-212), VH(2-113) and CH(114-213). | ||
| - | VH(Pro40 to Lys43) corresponds to a loop in the sequence between CDRs (Complementarity Determining Regions) heavy-chain 1 (H1) and H2, located in the hinge region of the Fab away from the ligand binding site. However, this loop is associated with poor electron density and, therefore, there is some uncertainty about the accuracy of the model in this region. | ||
| - | Ser62H is a non-CDR loop involved in symmetry-related close contacts. | ||
| - | Val51 of the light chain is the only residue to fall outside allowed regions of the Ramachandran plot. The unfavorable phi/psi torsion angles arise from the fact that this residue is in a γ-turn restrained by the (i to i+2) hydrogen bond between the Gln50L backbone carbonyl and the Ser52L amide. | ||
| - | + | Constant (C) and variable (V) domains of both light (<sub>L</sub>) and heavy (<sub>H</sub>) chains include the following residues : V<sub>L</sub>(1-107), C<sub>L</sub>(108-212), V<sub>H</sub>(2-113) and C<sub>H</sub>(114-213). | |
| + | <scene name='60/604482/My_first_scene/6'>Residues Pro40 to Lys43 of the variable heavy chain</scene> represent a loop in the sequence between Complementarity Determining Regions (CDR) heavy-chain 1 (H1) and H2, located in the hinge region of the Fab away from the ligand binding site. However, due to the poor electron density on this loop, some uncertainties about the accuracy of the model in this region can be found. <scene name='60/604482/My_first_scene/3'>Ser62H</scene> is a non-CDR loop involved in symmetry-related close contacts. | ||
| - | + | <scene name='60/604482/My_first_scene/5'>Val51L</scene> of the light chain is the only residue to stay outside from allowed regions of the Ramachandran plot. The unfavorable φ / Ψ torsion angles arise from the fact that this residue is in a γ-turn restrained by the (i to i+2) hydrogen bond between <scene name='60/604482/Scene_kerim_01/3'>the Gln50L backbone carbonyl and the Ser52L amide</scene>.<ref>Amyloid-beta-anti-amyloid-beta complex structure reveals an extended conformation in the immunodominant B-cell epitope.,Miles LA, Wun KS, Crespi GA, Fodero-Tavoletti MT, Galatis D, Bagley CJ, Beyreuther K, Masters CL, Cappai R, McKinstry WJ, Barnham KJ, Parker MW J Mol Biol. 2008 Mar 14;377(1):181-92. Epub 2008 Jan 30. PMID:18237744</ref> | |
| - | + | In the WO2 Form A structure, <scene name='60/604482/My_first_scene/12'>2 sodium ions Na+</scene> were found, involved in crystal contacts, and <scene name='60/604482/My_first_scene/15'>1 zinc ion Zn2+</scene> bound to Asp1L of WO2. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ===Details of the close interactions between WO2 and Aβ | + | |
| - | As mentionned previously, the residues of Aβ closely interacting with the CDRs of WO2 extend from Ala2 to Ser8. Let's focus on the interactions of each of these residues with the antibody : | + | ==Interactions with the ligand Aβ<sub>1-16</sub>== |
| + | ===Overview of the WO2:Aβ<sub>1-16</sub> complex=== | ||
| + | Aβ<sub>1-16</sub> represents the minimal zinc binding domain and contains the entire immunodominant B-cell epitope of Aβ, it is therefore interesting to see how this fragment of Aβ interacts with WO2. | ||
| + | |||
| + | The main residues which closely contact the CDRs of WO2 by sitting within the antigen binding site of WO2 are '''Ala2 to Ser8''' and they stretch 20 Å from the N-terminus to the C-terminus. | ||
| + | |||
| + | |||
| + | The surface area of the Aβ<sub>2-8</sub> structure is 1118 Ų, from which 60% is buried (665 Ų) in the antibody interface. In addition, we note two significant interfaces between Aβ and WO2 : 367 Ų of its surface contacts the heavy chain and 298 Ų contacts the light chain. We notice that residues in the middle of the Aβ<sub>1-16</sub> structure exhibit lower B-factors than atoms at the N- and C- terminus of the Aβ<sub>1-16</sub> peptide, indicating that they are more flexible (since the B-factor, also called the temperature factor, represents the relative vibrational motion of different parts of a structure and atoms with low B-factors belong to a part of the structure quite rigid whereas atoms with high B-factors generally belong to part of a structure that is very flexible[http://en.wikipedia.org/wiki/Debye%E2%80%93Waller_factor]). Phe4 and His6 are completely buried in the Fab interface, each with nearly half of their surface area buried in the V<sub>H</sub> interface and half in the V<sub>L</sub> interface. All other residues are located exclusively at the interface with either the V<sub>H</sub> or the V<sub>L</sub> domains. | ||
| + | |||
| + | Residues of the light chain closely contacting Aβ residues include <scene name='60/604482/His_27/2'>His27(D)L</scene>, <scene name='60/604482/Ser27/2'> Ser27(E)L </scene> and <scene name='60/604482/My_first_scene/18'>Tyr32L</scene> from light-chain CDR 1 (L1) and <scene name='60/604482/My_first_scene/7'>Ser92L, Leu93L, Val94L and Leu96L from L3</scene>. | ||
| + | All residues from Phe4 to Ser8, except Asp7, make close contact with the WO2 heavy-chain CDRs. Close contacting interface residues include <scene name='60/604482/My_first_scene/8'>His50H, Tyr52H, Asp54H and Asp56H from H2</scene> and <scene name='60/604482/My_first_scene/9'>Tyr100(B)H and Asn100(E)H from H3</scene>. | ||
| + | |||
| + | Also, we observe no contact between Aβ and the L2 or H1 CDRs of WO2 and there is no evidence in the structure of any water-mediated contacts between WO2 and Aβ.<ref>Amyloid-beta-anti-amyloid-beta complex structure reveals an extended conformation in the immunodominant B-cell epitope.,Miles LA, Wun KS, Crespi GA, Fodero-Tavoletti MT, Galatis D, Bagley CJ, Beyreuther K, Masters CL, Cappai R, McKinstry WJ, Barnham KJ, Parker MW J Mol Biol. 2008 Mar 14;377(1):181-92. Epub 2008 Jan 30. PMID:18237744</ref> | ||
| + | |||
| + | |||
| + | |||
| + | ===Details of the close interactions between WO2 and Aβ<sub>2-8</sub>=== | ||
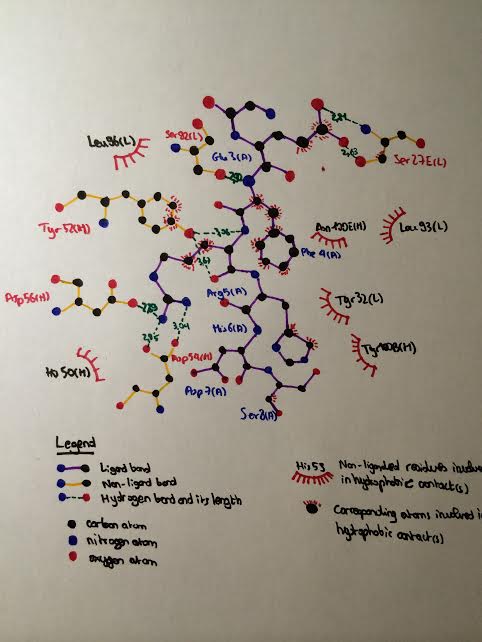
| + | As mentionned previously, the residues of Aβ closely interacting with the CDRs of WO2 extend from Ala2 to Ser8. Let's focus on the interactions of each of these residues with the antibody '''(see the following LIGPLOT scheme)''' : | ||
| + | [[Image:Ligplot.jpg|right|'''Figure 1''']] | ||
====Interactions with Ala2==== | ====Interactions with Ala2==== | ||
| - | Ala2 of is recognized by WO2 through a hydrogen bond between its main chain carbonyl and the amide of Val94L. | + | Ala2 of Aβ<sub>2-8</sub> is recognized by WO2 through a hydrogen bond between its main chain carbonyl group and the amide group of <scene name='60/604482/My_first_scene/4'>Val94L</scene>. |
====Interactions with Glu3==== | ====Interactions with Glu3==== | ||
| - | + | Hydrogen bond between the side chain of Glu3 and the main-chain amide of <scene name='60/604482/Ser27/2'> Ser27(E)L </scene> . Additionally, there are potential salt bridges between the side chain of Glu3 and Nδ1/Nε2 atoms of <scene name='60/604482/His_27/2'>His27(D)L</scene>. | |
====Interactions with Phe4==== | ====Interactions with Phe4==== | ||
| - | + | Hydrogen bond between the amide group of Phe4 and the carbonyl group of Ser92L. | |
====Interactions with Arg5==== | ====Interactions with Arg5==== | ||
| - | Arg5 is a donor for 5 side chain-side chain hydrogen bonds and 1 main chain-side chain hydrogen bond, involving Asp54H, Asp56H and Tyr52H | + | Arg5 is a donor for 5 side chain-side chain hydrogen bonds and 1 main chain-side chain hydrogen bond, involving <scene name='60/604482/Asp54h/1'> Asp54H </scene>, <scene name='60/604482/Asp56h/3'>Asp56H</scene> and <scene name='60/604482/Tyr_25h/1'>Tyr52H</scene> as shown below : |
| + | *The side chain of Arg5 forms : | ||
| + | **Two hydrogen bonds with the carboxylate group of <scene name='60/604482/Asp54h/1'> Asp54H </scene> | ||
| + | **One hydrogen bond with the side chain of <scene name='60/604482/Asp56h/3'>Asp56H</scene> | ||
| + | *The main chain of Arg5 interacts with the side-chain hydroxyl (OH) of <scene name='60/604482/Tyr_25h/1'>Tyr52H</scene> | ||
| + | |||
====Interactions with His6==== | ====Interactions with His6==== | ||
| - | His6 contacts both heavy- and light-chain elements with 10 side chain-side chain hydrophobic contacts with Asn100(E)H. Van der Waals contacts have been detected between His6 and Tyr32L of WO2. | + | His6 contacts both heavy- and light-chain elements with 10 side chain-side chain hydrophobic contacts with <scene name='60/604482/Asn100h/1'>Asn100(E)H</scene>. Van der Waals contacts have been detected between His6 and <scene name='60/604482/My_first_scene/18'>Tyr32L</scene> of WO2. |
With Phe4 and Arg5, His6 represents the core of the epitope of Aβ which sits at the heavy-chain/light-chain junction of the CDRs of WO2. | With Phe4 and Arg5, His6 represents the core of the epitope of Aβ which sits at the heavy-chain/light-chain junction of the CDRs of WO2. | ||
====Interactions with Ser8==== | ====Interactions with Ser8==== | ||
| - | Ser8 makes a single Van der Waals contact with Tyr100(B)H of WO2. | + | Ser8 makes a single Van der Waals contact with <scene name='60/604482/My_first_scene/10'>Tyr100(B)H</scene> of WO2. |
====The special case of Asp7==== | ====The special case of Asp7==== | ||
Asp7 sits above a large water-filled cavity in the Fab antigen binding site but makes no direct or water-mediated contacts with WO2. | Asp7 sits above a large water-filled cavity in the Fab antigen binding site but makes no direct or water-mediated contacts with WO2. | ||
| - | ===Comparison of | + | |
| - | + | ===Comparison of unliganded and liganded WO2 Fab structures=== | |
| - | Moreover, thanks to temperature-factors analysis, it appears that CDR H1 is | + | Unliganded and liganded structures superimpose very closely with an r.m.s.d. (root-mean-square deviation) of 0.3 Å on all Cα atoms (the r.m.s.d. is the measure of the average distance between the atoms of superimposed proteins[http://en.wikipedia.org/wiki/Root-mean-square_deviation]). Even the '''CDRs of liganded and unliganded states are barely distinguishable'''. Except for some small variations (<1 Å) around <scene name='60/604482/Ser27/2'> Ser27(E)L </scene> (L1), <scene name='60/604482/My_first_scene/11'>Lys33H (H1)</scene>, <scene name='60/604482/My_first_scene/16'>Asp54H (H2)</scene> and <scene name='60/604482/My_first_scene/17'>Glu100(C)H (H3)</scene>, there is no substantial change in the CDRs when Aβ binds with WO2. |
| + | Moreover, thanks to temperature-factors analysis, it appears that CDR H1 is less flexible in the liganded structure.<ref>Amyloid-beta-anti-amyloid-beta complex structure reveals an extended conformation in the immunodominant B-cell epitope.,Miles LA, Wun KS, Crespi GA, Fodero-Tavoletti MT, Galatis D, Bagley CJ, Beyreuther K, Masters CL, Cappai R, McKinstry WJ, Barnham KJ, Parker MW J Mol Biol. 2008 Mar 14;377(1):181-92. Epub 2008 Jan 30. PMID:18237744</ref> | ||
| + | |||
| - | == Biological | + | == Biological Relevance == |
| + | One of the most common isoforms of Aβ is the 42-mer Aβ (with the sequence : 1-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA-42), which is the most fibrillogenic isoforms and is, therefore linked to disease states. | ||
| - | + | Aβ<sub>42</sub> damages and kills neurons by generating reactive oxygen species when it self-aggregates. Aβ self-aggregation is promoted by its binding with metal ions (such as Cu<sup>2+</sup>, Zn<sup>2+</sup>, etc) by reason of, among others, its His6, His13, His14, Tyr10, Asp1 and Glu11 residues. If this process occurs on the neurons' membrane, it causes lipid peroxidation and the generation of a toxic aldehyde called 4-hydroxynonenal which, in return, impairs the function of ion-motive ATPases, glucose transporters and glutamate transporters. It also triggers depolarization of the synaptic membrane, excessive calcium influx and mitochondrial impairment, making neurons vulnerable to excitotoxicity and apoptosis : this is known as the beginning of the neurodegenerative process of AD.<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3091392/</ref> | |
| - | + | The important role of metals in AD is highlighted by a metal chelator, the clioquinol, which reduces amyloid plaques in the brain of AD patients. | |
| + | The mAb (monoclonal antibody) WO2 recognises the N-terminus of Aβ. This region of Aβ constitutes the immunodominant B-cell epitope of Aβ and lacks T-cell epitopes involved in the toxicity of previous clinical trials. Some experiments showed scientists that the great interest of WO2 lies in the fact that when it binds Aβ, it prevents Asp1 and His6 of Aβ to participate in metal coordination, preventing Aβ from aggregating and thus, neutralizing harmful effects of Aβ.<ref>Amyloid-beta-anti-amyloid-beta complex structure reveals an extended conformation in the immunodominant B-cell epitope.,Miles LA, Wun KS, Crespi GA, Fodero-Tavoletti MT, Galatis D, Bagley CJ, Beyreuther K, Masters CL, Cappai R, McKinstry WJ, Barnham KJ, Parker MW J Mol Biol. 2008 Mar 14;377(1):181-92. Epub 2008 Jan 30. PMID:18237744</ref> | ||
==Related Structures== | ==Related Structures== | ||
| - | + | *[[1plg]] : Another IgG<sub>2a</sub> κ murine mAb Fab | |
| - | + | *PFA1, PFA2 : Two other murine IgG<sub>2a</sub> mAbs, anti-protofibril antibodies<ref>http://www.pnas.org/content/104/40/15659.full</ref> | |
| - | + | *[[3bkc]] : WO2 Fab Form B | |
| - | + | *[[3bkj]] : WO2 Fab:Aβ<sub>1-16</sub> complex | |
| - | + | *[[3bae]] : WO2 Fab:Aβ<sub>1-28</sub> complex | |
==Contributors== | ==Contributors== | ||
| - | Kerim | + | Kerim Secener and Nicolas Pautrieux |
== References == | == References == | ||
| - | <references/ | + | <references/> |
Current revision
Anti-amyloid-beta Fab WO2 (Form A, P212121)
| |||||||||||