We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 954
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 9: | Line 9: | ||
Serpins are a superfamily of proteins wich are functionally distinct but structurally conserved. <ref>JBC Papers in Press. Published on July 2, 2001 as Manuscript R100016200 THE SERPINS ARE AN EXPANDING SUPERFAMILY OF | Serpins are a superfamily of proteins wich are functionally distinct but structurally conserved. <ref>JBC Papers in Press. Published on July 2, 2001 as Manuscript R100016200 THE SERPINS ARE AN EXPANDING SUPERFAMILY OF | ||
| - | STRUCTURALLY SIMILAR BUT FUNCTIONALLY DIVERSE | + | STRUCTURALLY SIMILAR BUT FUNCTIONALLY DIVERSE PROTEINS, http://www.jbc.org/content/early/2001/07/02/jbc.R100016200.full.pdf DOI : 2001/07/02/jbc.R100016200.full.pdf </ref> |
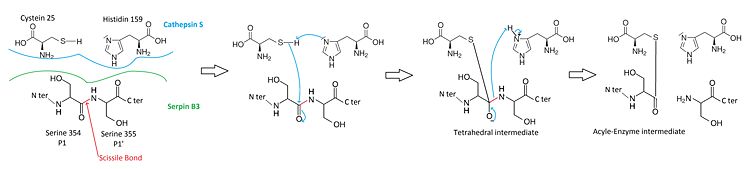
SerpinB3 means serin protease inhibitor, clade B (for ovalbumin), member 3. The particularity of serpin B3 is to target proteases which have a nucleophilic cysteine instead of serine in their catalytic site. | SerpinB3 means serin protease inhibitor, clade B (for ovalbumin), member 3. The particularity of serpin B3 is to target proteases which have a nucleophilic cysteine instead of serine in their catalytic site. | ||
SCCA1 is a <scene name='60/604473/Trimeric/1'>trimeric protein</scene><ref> PMID : 19166818 </ref>. <scene name='60/604473/One_subunit/1'>One subunit</scene> has three β sheets termed <scene name='60/604473/A_beta_sheet/3'>A (7 stranded)</scene>, <scene name='60/604473/B_beta_sheet/2'>B (5 stranded)</scene> and <scene name='60/604473/C_beta_sheet/2'>C (6 stranded)</scene> and <scene name='60/604473/Alpha_helices/1'>11 α helices (hA to hK)</scene> <ref>Gary A. Silverman1*, Phillip I. Bird2 | SCCA1 is a <scene name='60/604473/Trimeric/1'>trimeric protein</scene><ref> PMID : 19166818 </ref>. <scene name='60/604473/One_subunit/1'>One subunit</scene> has three β sheets termed <scene name='60/604473/A_beta_sheet/3'>A (7 stranded)</scene>, <scene name='60/604473/B_beta_sheet/2'>B (5 stranded)</scene> and <scene name='60/604473/C_beta_sheet/2'>C (6 stranded)</scene> and <scene name='60/604473/Alpha_helices/1'>11 α helices (hA to hK)</scene> <ref>Gary A. Silverman1*, Phillip I. Bird2 | ||
| Line 30: | Line 30: | ||
===Conformational changes of serpins=== | ===Conformational changes of serpins=== | ||
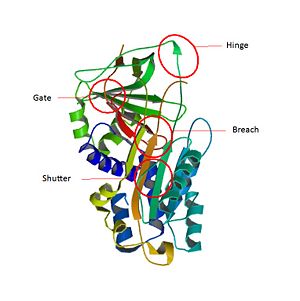
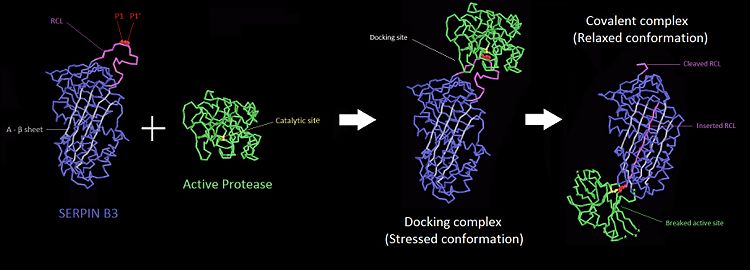
| - | The inhibitory members of serpin family undergo an unusual conformational change, the Stressed to Relaxed transition. This structural transition causes the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> insertion into <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene> thereby the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> forms an extra β strand. The serpin conformational change is essential for the inhibitor mechanism of proteases. <scene name='60/604473/Rcl_insertion_into_beta_sheet/1'>Some amino-acids of RCL</scene> wich belong to a consensus sequence for inhibitory serpins are thought to permit the insertion of the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> into the <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene>.<ref> James C Whisstocka, 2, Richard Skinnera, 2, Robin W Carrella, Arthur M Leska, Conformational changes in serpins: I. the native and cleaved conformations of α1-antitrypsin1, http://www.sciencedirect.com/science/article/pii/S0022283699935209 DOI: | + | The inhibitory members of serpin family undergo an unusual conformational change, the Stressed to Relaxed transition. This structural transition causes the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> insertion into <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene> thereby the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> forms an extra β strand. The serpin conformational change is essential for the inhibitor mechanism of proteases. <scene name='60/604473/Rcl_insertion_into_beta_sheet/1'>Some amino-acids of RCL</scene> wich belong to a consensus sequence for inhibitory serpins are thought to permit the insertion of the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> into the <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene>.<ref> James C Whisstocka, 2, Richard Skinnera, 2, Robin W Carrella, Arthur M Leska, Conformational changes in serpins: I. the native and cleaved conformations of α1-antitrypsin1, http://www.sciencedirect.com/science/article/pii/S0022283699935209 DOI:10.1006/jmbi.1999.3520</ref>Key regions able to control and modulate the conformational change of RCL. The hinge which is the <scene name='60/604473/P9-p15/1'>P15-P9 portion of the RCL</scene> is responsible for the mobility which is essential during the conformational change in the Stress to Relax transition. The breach is situated in the top of the <scene name='60/604473/A_beta_sheet/3'>β-sheet</scene>. It is located at the point of initial insertion of the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> into the <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene>. The shutter is next to the <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene>. It facilitates the beta-sheet opening and accept the conserved hinge of the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> as it insert. The gate is fully inserted into the <scene name='60/604473/A_beta_sheet/3'>A β-sheet</scene> without cleavage, the <scene name='60/604473/The_rcl_loop_scene/3'>RCL</scene> has to pass around the β-turn linking strands. |
| - | [[Image:Structure region.jpg|center|thumbnail|300px|'''Key regions able to control and modulate the conformational change of RCL''']] | + | |
| + | [[Image:Structure region.jpg|center|thumbnail|300px|'''Key regions able to control and modulate the conformational change of RCL<ref>PMID :11116082</ref>''']] | ||
==Main SCCA1 function== | ==Main SCCA1 function== | ||
| Line 102: | Line 103: | ||
= Disease = | = Disease = | ||
| - | Asthma is characterized by an obstruction of the interior respiratory tract and an excessive mucus secretion.<ref>Santé médecine, Hyperplasie définition[http://sante-medecine.commentcamarche.net/faq/13479-hyperplasie-definition DOI : faq/13479-hyperplasie-definition]</ref> Experiments were performed on mice, mice lacking | + | Asthma is characterized by an obstruction of the interior respiratory tract and an excessive mucus secretion.<ref>Santé médecine, Hyperplasie définition[http://sante-medecine.commentcamarche.net/faq/13479-hyperplasie-definition DOI : faq/13479-hyperplasie-definition]</ref> Experiments were performed on mice, mice lacking serpinB3 showed a decrease of the mucus secretion. As a result serpinB3 may have a role in mucus hypersecretion in a house dust mist model of asthma. The SPDEF (SAM pointed domain containing ETS transcription factor) expression causes the hyperplasia of goblet cell. The hyperplasia designates the abnormal augmentation of cells number in a tissue, the subexpression of goblet cells m ay induce cancer. Serpin B3 increase SPDEF expression and goblet cells hyperplasia. <ref> PMID: 3058372 </ref> |
= Regulation = | = Regulation = | ||
| - | The E-cadherin can regulate the SCCA1 production in the squamous cell carcinoma of the uterin cervix. E-cadherins are transmembrane proteins, they have a role in cell adhesion because they are able to form adherens junctions. They have to bind a Ca++ ion to work. Using an anti-E-cadherin antibody induces the dissociation of the cervical squamous cell carcinoma. It also induces a decrease of | + | The E-cadherin can regulate the SCCA1 production in the squamous cell carcinoma of the uterin cervix. E-cadherins are transmembrane proteins, they have a role in cell adhesion because they are able to form adherens junctions. They have to bind a Ca++ ion to work. Using an anti-E-cadherin antibody induces the dissociation of the cervical squamous cell carcinoma. It also induces a decrease of SCCA1 in the cytosol and SCCA1 mRNA. Besides the phosphatidyl inositol 3 kinase is a mediator of E-cadherin. The E-cadherin mediates cell-cell adhesion and maintains SCCA1 production thanks to phosphatidyl inositol 3 kinase in squamous cell carcinoma.<ref> PMID: 14719077 </ref> |
| Line 112: | Line 113: | ||
= References = | = References = | ||
<references/> | <references/> | ||
| - | |||
| - | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | ||
Current revision
SQUAMOUS CELL CARCINOMA ANTIGEN 1
| |||||||||||