This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Antimicrobial peptides
From Proteopedia
| (17 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == | + | ==Antimicrobial peptides== |
| - | <StructureSection load='3rec' size='350' side='right' caption='' scene='67/676980/4mgp_ala-mag/1'> | + | <StructureSection load='3rec' size='350' side='right' caption='Magainin 2 peptide (PDB code [[4mgp]])' scene='67/676980/4mgp_ala-mag/1'> |
| Line 114: | Line 114: | ||
Although AMPs have the same effect on the cell mambrane, they do not seem to have the same structure. we can find a big variety of structures among familiar AMPs. | Although AMPs have the same effect on the cell mambrane, they do not seem to have the same structure. we can find a big variety of structures among familiar AMPs. | ||
| - | (1) Some have '''helical structures''', for some of the peptide sequence, such as Magainin | + | (1) Some have '''helical structures''', for some of the peptide sequence, such as ,<scene name='67/676980/Magainin/1'>Magainin</scene>, or a helical structure throughout the whole peptide, such as <scene name='67/676980/Magainin_2_simple/1'>Magainin 2</scene>. this peptide was found on a frog's skin. you can see the page about Magainin2 here : [[Magainin 2]]. |
(2) '''Beta-sheet structures''': | (2) '''Beta-sheet structures''': | ||
| Line 128: | Line 128: | ||
==Suggested Mechanisms== | ==Suggested Mechanisms== | ||
| - | The | + | For killing the bacteria, AMPs must first attracted to bacterial surfaces. The attraction is electrostatic bonding between anionic or cationic peptides and structures on the bacteria's membrane. Studies on membrane models contained vary compositions of charged phospholipids proved this mechanism of attraction. But, bacteria membranes are more complex than mebrane models. Gram negative - Cationic AMPS suggested to be attracted to the net negative charge on the puter mmebrane (phospholipids to phosphate groupf of lipopolysacharides). Gram positive - AMPSs suggested to be attracted to the trichoic acids ob the surface. |
| + | Now, the peptides are attached to the cell. However they have to tranverse the lipopolysacharides or extracellular polysacharides so they can contact and act on the outer membrane. the specific mechanism of this step is unclear so far. | ||
| - | There are a few suggested machanisms of how AMPs work(William C. Wimley, ACS CHEMICAL BIOLOGY, 2010). | + | |
| + | The way how different antimicrobial peptides insert and act on the membrane goal appears to be different and depends on the peptide, the membrane and the peptide/lipid ratio. | ||
| + | |||
| + | At low peptide/lipid ratios, peptides are oriented parralel to the lipid bilayer, | ||
| + | As the peptide/lipid ration increases, peptides are oriented prependicular to the membrane and insert inside it, forming transmembranes pores. | ||
| + | |||
| + | There are a few suggested machanisms of how AMPs work (William C. Wimley, ACS CHEMICAL BIOLOGY, 2010). | ||
They can be divided into two: | They can be divided into two: | ||
(A) Transmembrane Pore Models of AMP Membrane Activity and (B) Nonpore Models of AMP Activity | (A) Transmembrane Pore Models of AMP Membrane Activity and (B) Nonpore Models of AMP Activity | ||
In the Transmembrane Pore Models, it is suggested that AMPs form many pores in the mambrane, so that it cannot hold it's content anymore. | In the Transmembrane Pore Models, it is suggested that AMPs form many pores in the mambrane, so that it cannot hold it's content anymore. | ||
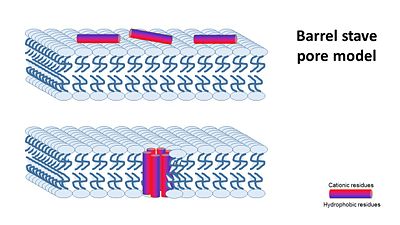
| - | + | The''' transmembrane pore mechanism''' has 2 main models: | |
| - | + | ||
| - | + | ||
| - | + | '''1- barrel stave pore model''' ,that claims peptides interact laterally with one another to form a specific structure enclosing a water-filled channel, much like a protein ion channel. | |
| - | [[Image: | + | [[Image:Barrel stave pore model.JPG|center|400px]] |
| - | the Nonepore model claims peptides bind to the membrane until it collapses. It is devided into 2 main mechanisms: | ||
| - | 1- The carpet model. In this model, antimicrobial peptides accumulate on the membrane surface with an orientation that is parallel to the membrane.When peptide concentration has reached a critical level permeabilization occurs via global bilayer destabilization. | ||
| - | 2- detergent model- collapse of membrane integrity, observed with some AMPs at high peptide concentration. | ||
| - | [[Image:Detregent_model.JPG]] | ||
| - | |||
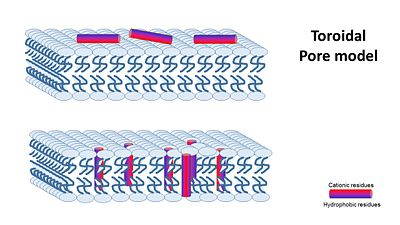
| + | '''2- toroidal pore model''', that claims specific peptide–peptide interactions are not present, and instead, single peptides are bound to the membrane’s phospholipids and disturbe it’s structure.. | ||
| + | [[Image:Torodial pore model.JPG|center|400px]] | ||
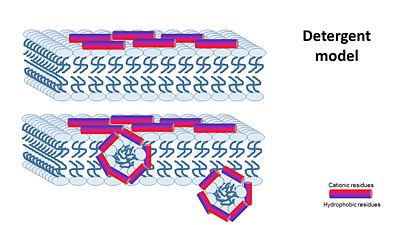
| - | + | the '''Nonepore model''' claims peptides bind to the membrane until it collapses. It is devided into 2 main mechanisms: | |
| + | '''1- The carpet model''': In this model, antimicrobial peptides accumulate on the membrane surface with an orientation that is parallel to the membrane.When peptide concentration has reached a critical level permeabilization occurs via global bilayer destabilization. | ||
| + | '''2- detergent model''' : collapse of membrane integrity, observed with some AMPs at high peptide concentration. | ||
| + | [[Image:Detergent model.JPG|center|400px]] | ||
| + | |||
| + | == Relevance == | ||
| + | |||
| + | [[magainin 2]] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | 1- '''J. Gesella., M. Zasloffb and S. J. Opellaa'''., Two-dimensional H NMR experiments show that the 23-residue magainin antibiotic peptide is an α-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. ''Journal of Biomolecular NMR'', 1997. 9: 127–135 | ||
| + | |||
| + | 2- '''Z. Hayouka., D. E. Mortenson., D. F. Kreitler., B. Weisblum., K. T. Forest, and S. H. Gellman'''., Evidence for Phenylalanine Zipper-Mediated Dimerization in the X‑ray Crystal Structure of a Magainin 2 Analogue. ''J. Am. Chem. Soc'', 2013. 135: 15738−15741. | ||
| + | |||
| + | 3- '''T. Nakatsuji., & R. L. Gallo'''., Antimicrobial Peptides: Old Molecules with New Ideas. ''Journal of Investigative Dermatology'', 2012. 132: 887-895. | ||
| + | |||
| + | 4- '''K. A. Brogden'''., ANTIMICROBIAL PEPTIDES: PORE FORMERS OR METABOLIC INHIBITORS IN BACTERIA?. ''NATURE REVIEWS MICROBIOLOGY'', 2005. 3: 238-250. | ||
| + | |||
| + | 5- '''W. C. Wimley'''., Describing the Mechanism of Antimicrobial | ||
| + | Peptide Action with the Interfacial Activity Model. ''ACS CHEMICAL BIOLOGY'', 2010. 10: 905-917. | ||
| + | |||
| + | 6- '''W. C. Wimley., K. Hristova'''., Antimicrobial Peptides: Successes, Challenges and Unanswered | ||
| + | Questions. ''Membrane Biol'' ,2011. 239: 27–34. | ||
| + | |||
| + | |||
| + | |||
<references/> | <references/> | ||
Current revision
Antimicrobial peptides
| |||||||||||
References
1- J. Gesella., M. Zasloffb and S. J. Opellaa., Two-dimensional H NMR experiments show that the 23-residue magainin antibiotic peptide is an α-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. Journal of Biomolecular NMR, 1997. 9: 127–135
2- Z. Hayouka., D. E. Mortenson., D. F. Kreitler., B. Weisblum., K. T. Forest, and S. H. Gellman., Evidence for Phenylalanine Zipper-Mediated Dimerization in the X‑ray Crystal Structure of a Magainin 2 Analogue. J. Am. Chem. Soc, 2013. 135: 15738−15741.
3- T. Nakatsuji., & R. L. Gallo., Antimicrobial Peptides: Old Molecules with New Ideas. Journal of Investigative Dermatology, 2012. 132: 887-895.
4- K. A. Brogden., ANTIMICROBIAL PEPTIDES: PORE FORMERS OR METABOLIC INHIBITORS IN BACTERIA?. NATURE REVIEWS MICROBIOLOGY, 2005. 3: 238-250.
5- W. C. Wimley., Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS CHEMICAL BIOLOGY, 2010. 10: 905-917.
6- W. C. Wimley., K. Hristova., Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. Membrane Biol ,2011. 239: 27–34.