|
|
| (149 intermediate revisions not shown.) |
| Line 2: |
Line 2: |
| | ==Histone Deacetylase 8== | | ==Histone Deacetylase 8== |
| | <StructureSection load='1t64' size='340' side='right' caption='Caption for this structure' scene=''> | | <StructureSection load='1t64' size='340' side='right' caption='Caption for this structure' scene=''> |
| - | This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs.
| + | Chromatin, in eukaryotes, is a very compact structure mainly due to the electrostatic interaction between DNA and [http://proteopedia.org/wiki/index.php/Histone histones] <ref>Ramakrishnan, V. Histone Structure and the Organization of the Nucleosome. Annual Review of Biophysics and Biomolecular Structure 26, 83–112 (1997).</ref>. This interaction happens because DNA carries an overall negative charge and the histones carry a positive charge when deacetylated. In order to carry out the basic functions, such as transcription and replication, the chromatin has to be decondensed so that enzymes and transcription factors can access the DNA. The decondensation is mainly carried out by the acetylation of a lysine residue on the histone tail by [http://en.wikipedia.org/wiki/Histone_acetyltransferase Histone Acetyl Transferases] (HATs). This then neutralizes its positive charge. The removal of the acetyl group is carried out by different enzyme family the [http://proteopedia.org/wiki/index.php/HDAC histone deacetylases] (HDACs). |
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue.
| + | [[Image:Acetylation_and_deacetylation_of_the_lysine_residue.jpg|300px|left|thumb| '''Fig. 1''' Acetylation and deacetylation of the lysine residue by HATs and HDACs, respectively.]] |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | | | |
| | == Function == | | == Function == |
| - | Alex is a <scene name='69/699997/Structure/1'>skank</scene>
| + | HDAC8 contains a [http://en.wikipedia.org/wiki/Nuclear_localization_sequence nuclear localization signal] (NLS) at the center of the catalytic domain, and because of this tag it is found in the nucleus, however, it has been reported that HDAC8 has a cytosolic localization in muscle cells <ref>Waltregny, D. et al. Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essential for smooth muscle cell contractility. FASEB J. 19, 966–968 (2005).</ref>. Global deletion of HDAC8 in mice leads to prenatal death due to instability of the skull <ref>Haberland, M., Mokalled, M. H., Montgomery, R. L. & Olson, E. N. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 23, 1625–1630 (2009).</ref>. HDACs seem to serve an important function in learning, memory and cognition in humans <ref>Gräff, J. & Tsai, L.-H. The Potential of HDAC Inhibitors as Cognitive Enhancers. Annual Review of Pharmacology and Toxicology 53, 311–330 (2013).</ref>. |
| - | <scene name='69/699997/Monomer/1'>Monomer</scene>
| + | |
| - | <scene name='69/699997/Tsa_binding/1'>TSA_Binding</scene>
| + | |
| - | Function
| + | |
| - | HDAC8 was first cloned and characterized by three independent research groups [28-30]. It has several unique features differentiating it from other HDAC isozymes. HDAC8 contains a nuclear localization signal (NLS) at the center of the catalytic domain, and it has been reported to be localized in nucleus. However, David Waltregny et al. reported the cytosolic localization of HDAC8 in muscle cell [31]. Two transcript variants of different length namely 2.0 kb and 2.4 kb have been identified, which are produced due to an alternative splicing of the RNA primary transcript. HDAC8 does not associate with any co-repressors; presumably, due to the lack of an extra C-terminal region [32]. Unlike HDAC1 and 2, the catalytic activity of HDAC8 is reduced upon phosphorylation at Ser 39 residue localized in the catalytic domain [33]. Haberland et al. reported that the global deletion of HDAC8 in mice leads to prenatal death due to instability of skull [34]. The HDAC8 expression level has been correlated with neuroblastoma tumorigenesis where an HDAC8 selective inhibitor has potential to alleviate the disease condition [35]. In Cornelia de Lange syndrome, the cohesin acetylation cycle has been reported to be impaired due to mutations in the HDAC8 gene [36]. The enzyme activity of Class I HDACs including HDAC8 has been found to be reduced in Chronic Obstructive Pulmonary Disease (COPD) [37]. HDAC8 forms a complex with protein phosphatase 1 (PP1), which leads to the inactivation of CREB mediated transcription. Both HDAC1 and HDAC8 are involved in dephosphorylation of Ser 133 of CREB in association with PP1 [38]. Durst et al. have reported that the inv (16) produces an oncogenic fusion protein (CBFβ-SMMHC) in acute myloid leukemia, which reportedly associates with HDAC8 [39]. Their interaction is disrupted in the presence of an HDAC8 inhibitor, suggesting the therapeutic utility of an HDAC8 selective inhibitor in acute myeloid leukemia.
| + | |
| - | Disease
| + | |
| - | HDACs are high priority drug target for the treatment of several human diseases including cancer [95]. Towards this end, serious attempts have been made to discover their small molecule effectors, which could potentially alleviate the disease condition. HDACs have been found to overexpresed in various forms of human cancer [95]. HDAC1 is primarily overexpresed in gastric, prostrate, colon, and breast cancer [95, 96]. The expression of HDAC2 is elevated in breast, colon, colorectal and cervical cancer [97]. An overexpression of HDAC3 has been linked with breast and colon cancer [98]. Notably, among all HDAC isozymes, an exclusive overexpression of HDAC8 has been reported in neuroblastoma cell [99].
| + | |
| - | Serious attempts have been made by several research groups to elucidate the link between HDAC and cancer. A global loss of H4 acetylation at Lys 16 has been reported in various forms of cancer, suggesting that histone modification is closely link to cancer [100]. Class I HDAC isozymes are reportedly involved in expression p21, a cyclin-dependent kinase inhibitor [101]. The p21 primarily inhibits an uncontrolled cell proliferation, and thereby helps to prevent/eliminate the malignant condition in human cell/tissue.
| + | |
| - | Several non-histone targets of HDACs, such as p53, NFB, etc., are intimately linked with cancer initiation and progression pathways [102]. A mutation in p53 gene has been widely reported in cancer patients [103]. HDAC1 and 2 have been found to reduce the expression of cadherin-E, which plays a crucial role cancer metastasis. HDACs are recruited to several fusion proteins, such as RAR-PML, RAR-PLZF, which are intimately linked with various forms of hematological malignancies [104]. Recently, HDAC8 has been shown to regulate the expression of both the wild type and the mutant form of p53 in a HoxA dependent manner [103].
| + | |
| - | An inhibition of HDAC has been known to induce an anticancer affect both in vitro as well as in xenograft animal model [105]. However, a pan-HDAC inhibitor usually shows a considerable side affect in a clinical setting, primarily because of an indiscriminate inhibition of the multiple HDAC isozymes involved in several vital cellular processes. In view of the above fact, serious attempts are being made to understand the roles of each HDAC isozyme in a specific form of cancer, so that it can be selectively targeted by an isozyme selective HDAC inhibitor. Notably, isozyme selective inhibitors are likely to have a lower side effect as compared to a pan-inhibitor.
| + | |
| - | In addition to malignancy, HDACs have been found to regulate learning, memory and cognition in human [106]. Several HDAC isozymes are widely expressed in brain tissues. HDAC2 expression level is reduced in nucleus accumbons region of human brain afflicted with a chronic depression [107]. Li-Hui Tsai and co-workers reported the role of HDAC2 in regulation of memory and synaptic plasticity [108]. Valproic acid, an HDAC inhibitor, has been widely used for the treatment of psychiatric disorders [109]. Mutation in HDAC8 is linked with Cornelia de Lange syndrome, where the cognitive behavior of children is severely affected [36]. Furthermore, HDAC inhibitors have shown promising results in ameliorating the neurodegenerative conditions associated with Alzheimer and Hutchinson diseases [109].
| + | |
| - | Recently, HDACs have emerged as therapeutic target for the treatment of heart diseases [110]. Joseph A. Hill and co-workers discovered the role of HDAC1 and 2 in autophagy mediated cardiac hypertrophy [111]. HDAC4, 5 and 9 are reportedly involved in cardiac hypertrophy in an MEF-2-dependent manner. Timonthy has reviewed the therapeutic potential of HDAC inhibitors for the treatment of symptoms associated with heart failure [110]. In addition to cancer and heart disease, HDACs are linked with several inflammatory diseases, such as chronic obstructive pulmonary disease (COPD) and rheumatoid arthritis [112]. In COPD, HDAC enzyme activity is reportedly low [113].
| + | |
| - | HDACs inhibitors have been widely known to have an anticancer affect. They induce growth inhibition, dedifferentiation, and even cell death in cancer cell both in vitro as well as in a xenograft animal model [72]. Attempts have been made to elucidate the molecular mechanism of anticancer effect mediated via an HDAC inhibitor. It is important to note that, an HDAC inhibitor such as SAHA influences the expression of only 2-10 % of the total genes in cancer cell [114]. Surprisingly, normal cells essentially remain unaffected upon the treatment with same dosage of inhibitor. An HDAC inhibitor modulates the expression of various genes which are primarily involved in cell cycle, differentiation and caspase-mediated cell death [115]. Several HDAC inhibitors have been reported to down-regulate the expression of an angiogenic factor (VGFG) primarily involved in cancer metastasis, rationalizing the utility of an HDAC inhibitor for the treatment of metastatic cancer [116]. Additionally, the anticancer effect of an HDAC inhibitor has also been found to be mediated via an immunomodulation. Thomas B. Tomasi and co-workers reported that an immunomodulatory effect of an HDAC inhibitor in cancer cell, which is primarily due to the elevated expression of major histocompatibility complex (MHC I and MHC II) and CD40 [117]. The molecular mechanisms by which an HDAC inhibitor influences the expression of few selected genes are not yet understood [118]. However, it is widely known that a pan-HDAC inhibitor significantly affects the acetylation status of its histone as well as several non-histone proteins, such as HSP90, p53, tubulin, etc. Notably, the anticancer effect of an HDAC inhibitor is also dependent inhibitor-type and the nature of cancer cell, suggesting that underlying molecular mechanism of anticancer effect is different in various forms of cancer [119,120].
| + | |
| - | HDAC inhibitors have been shown to be very effective for the treatment various hematological malignancy [72]. However, their therapeutic efficacy is reportedly low, and they are ineffective against solid tumors [121]. A combination therapy utilizing an HDAC inhibitor along with other anticancer drugs has been proposed, which is likely to enhance the efficiency of cancer therapy in various forms of cancer including solid tumors. Ravi Bhatia and co-workers have reported an enhanced efficacy of an HDAC inhibitor in reducing the number leukemic stem cells, when it is used in combination with an another anticancer drug, Imatinib Mesylate [122]. Notably, cancer stem cells essentially remain unaffected by an HDAC inhibitor alone, often leading to a relapse/remission of malignancy after chemo and/or radiotherapy.
| + | |
| - | In recent years, it has been realized that targeting a specific form of cancer by isozyme selective inhibitor of HDAC would boost up the efficiency of cancer therapy, especially because of their limited side effects [123]. However, the above approach has the following limitations. Firstly, HDAC isozymes in human share a high degree of similarity in the geometry of their catalytic pocket, which limits the development of an isozymes selective inhibitor. Secondly, the link between a specific HDAC isozyme with a particular form of cancer is not well defined.
| + | |
| - | Therapeutic efficacy of HDAC inhibitor in vivo is difficult to predict a priori. It has been widely known that even the structurally similar HDAC inhibitors show a differential effect on the same cancer cell/tissue. P. G. Parson and co-workers investigated the antitumor activity of selected hydroxamate-based inhibitors, namely Alelaic bishydroxamate (ABHA) and Trichostatin A (TSA), in vitro and in a xenograft animal model. Both the above inhibitors showed a similar anticancer effect in vitro, while the TSA failed to exhibit any effect in vivo [119]. Furthermore, in a study performed by J. Chang et al. TSA and Romidepsin, which contain an unrelated Zn-binding group, were found to induce a different cellular response even within the same cancer cell line [120]. In view of the above facts, it is now widely realized that it is difficult to assess the therapeutic efficiency of an HDAC inhibitor based on its in vitro inhibitory potency.
| + | |
| - | Therapeutic potential of HDAC activators have not been well understood so far. However, there are several human diseases where an HDAC activator could be of great therapeutic benefits. In COPD and CLdS where the HDAC enzyme activity has been reported to be reduced, HDAC activators have a potential to ameliorate the disease conditions [113, 36]. Based on a recent study performed by X. Chen and co-worker, therapeutic potential of an HDAC8 selective activator for the treatment of various forms of cancers has recently emerged. In the above case the expression of the tumor suppressor protein (p53) is reportedly suppressed by HDAC8 [103].
| + | |
| - | Therapeutic efficacy of several HDAC inhibitors has been widely evaluated in clinical settings [141]. Table 1.5 shows the list of HDAC inhibitors which are at different stages of clinical trials for the treatment of various forms of cancer. Notably, SAHA (Zoliza) and Romidepsin (Istodax) have already been approved by FDA for the treatment of cutenous T-cell lymphoma [141]. The HDAC inhibitors listed in the above table are usually classified into the following four classes: hydroxamate, short chain fatty acid, benzamaide, and cyclic peptide. The hydroxamate based inhibitors usually have high potency (nanomolar range). However, they usually show a considerable toxicity in vivo. Romedepsin is a cyclic peptide inhibitor and reportedly shows an IC50 in a nanomolar range. Benzamide inhibitors, such as Entinostat (SNDX-275), Mocetinostat (MS27-275), have been found to be relatively selective toward class I HDAC inhibition. Short chain fatty inhibitors, such as valproic acid, phenylbutyrate have a low inhibitory potency.
| + | |
| - | Therapeutic efficacy of an HDAC inhibitor in clinical settings reportedly depends on its biophysical properties, interaction with receptor/target, bioavailability, pharmacokinetics and pharmacodynamics, as well as on the genotype of a cancer cell, where it is tested [141]. The above properties of an inhibitor are primarily dependent on its chemical structure/nature. Additionally, the side effect an HADC inhibitor is often dependent on its class. For example, hydroxamate-based drugs usually show higher side effects than the others [124]. Even within a class, a therapeutic efficacy has been found to be very different [119]. In view of the above fact, there has been an ongoing effort to develop novel HDAC inhibitors which would show a better therapeutic efficacy in clinic than the currently known inhibitors. Veronica Novoty-Diermayr and co-workers evaluated the efficacy of the two structurally similar hydroxamate inhibitors, namely, SAHA and SB939, and reported the following major differences [142]. The bioavailability and half-life of SB939 was significantly higher (3-4 fold) than SAHA, which is likely to be translated into a higher efficacy of the former in vivo as well as in a xenograft animal model. Thomas Becker et al. reported the class specific differences in the pharmacological properties of HDAC inhibitors, such as hydroxamate vs. benzamide [143]. Clarie Bonfils et al. developed an in vivo enzyme assay utilizing a fluorogenic substrate and validated its utility for studying the pharmacodynamics of MGCD0103 [144]. Interestingly, a change in the expression level of an HDAC has been found to influence the in vivo efficacy of HDAC inhibitors. Debdutta et al. reported that an overexpression of HDAC1 in cancer cells produces a drug resistance against sodium butyrate [145]. Furthermore, truncation mutation in HDAC2 is linked with the drug resistance in cancer cells against several HDAC inhibitors. More recently the presence of a biomarker, HR32B, which is involved in shuttling a cargo to proteosome, has been reported to influence the therapeutic efficacy of an HDAC inhibitor in cancer cells [141].
| + | |
| - | Structural highlights
| + | |
| - | Crystal structure of human HDAC8
| + | |
| - | The crystal structures of human HDAC8 have been primarily solved by two independent research groups [57, 58]. HDAC8 was the first among the HDAC isozymes whose crystal structure became available. Figure 1.2 shows the ribbon structure of HDAC8 bound with a canonical hydroxamate inhibitor, SAHA (Suberoylanilide Hydroxamic Acid) [pdb 1T69]. HDAC8 contains a single α/β-deacetylase domain consisting of 13 α-helices and an eight-stranded parallel β-sheet. Multiple loops, which emanate from the protein core, play a significant role in maintaining the appropriate geometry of the catalytic pocket. The α/β-fold of the above kind was first observed in a metalloenzyme, Arginase. The active site of HDAC8 contains a tubular cavity leading to catalytic machinery at the end. The catalytic machinery is comprised of a Zn2+ ion penta-coordinated with a square pyramidal geometry. The residues His 180, Asp 267 and Asp 176 occupy the three co-ordination sites, whereas the hydroxamate moiety of SAHA occupies the remaining two sites. In addition, the carbonyl oxygen of the hydroxamate moiety forms a hydrogen bond with Tyr 306. In the absence of any ligand (substrate/inhibitors), two water molecules are bound to the Zn2+ ion. The linker domain of the inhibitor interacts with the residues, namely, Phe 152, Phe 208, His 180, Gly 151, and Met 274, which forms a hydrophobic tunnel. Notably, the above residues involved in the inhibitor/substrate binding, as well as the catalysis, have been found to be conserved during the course of evolution among class I HDACs. The crystal structure of a mutant (Y306A) and catalytically inactive form of HDAC8 bound with a p53-derived deacetylated peptide substrate (pdb 2V5W) has been solved by Vannini et al. (Figure 1.3) [59]. The structure is very similar to the HDAC8-inhibitor complex reported previously except for the mode of binding of the ligand. The carbonyl moiety of the acetyl-lysine binds to the catalytic Zn2+ in a monodentate fashion by removing one of the zinc-bound water molecules. However, the hydroxamate moiety of a competitive HDAC inhibitor binds to the Zn2+ ion in a bidentate fashion, removing both of the Zinc-bound water molecules. Notably, a single Zinc-bound water molecule present in the HDAC8-substrate complex serves as a nucleophile in the deacetylation reaction. In addition, it makes hydrogen bonding with the N2 atom of imidazole of His 142 and His 143, which reportedly form a charge-relay system, respectively, with Asp 176 and Asp 183. The charge-relay system enhances the basicity of the N2 of the imidazole and has been reported previously in serine proteases [57]. Notably, the carbonyl oxygen of the acetyl-lysine substrate forms a hydrogen bond with the hydroxyl moiety of Y306.
| + | |
| - | Aside from the catalytic Zn2+ ion, the enzyme activity of HDAC8 is dependent on the presence of the monovalent ion, K+/Na+ [60]. The crystal structure of HDAC8 shows the presence of two binding sites for K+ (Figure 1.4) [58]. The first K+ binding site (K1) is located in the vicinity of the enzyme catalytic machinery, and it is hexacoordinated (octahedral geometry) with His 180 (carbonyl oxygen of the main chain), Asp 176 (oxygen atom of the main chain and side chain), Leu 200 (carbonyl oxygen of the main chain), and Ser 199 (O). Notably, His 180 and Asp 176 are the common residues coordinated with both the catalytic Zn2+ as well as the K+ ion. The second binding site for K+ ion (K2) is located 15Å away from the catalytic Zn2+ ion. It is hexacoordinated (octahedral geometry) with F189, T192, V195, Y225 as well as two water molecule (Figure 1.4). Gantt et al. reported that the binding of a K+ ion to K1 and K2, respectively, leads to the inhibition and activation in HDAC8 deacetylase activity [60]. Additionally, the presence of K+ ion at K1 site neutralizes the positive charge of tetrahedral oxyanion intermediate produced during the deacetylation reaction. It is important to note that the HDAC8 residues binding to the K+ ions are strictly conserved among class I HDACs [58].
| + | |
| - | The crystal structure of HDAC8-substrate complex (pdb 2V5W) elucidates the role of an aspartate residue (D101) in the substrate binding [59]. Asp 101 resides on the L2 loop and its carboxylate moiety makes two consecutive hydrogen bonds with the backbone of the p53-derived deacetylated peptide substrate as shown in Figure 1.3. Mutation of Asp 101 to Ala abolishes the HDAC8 catalytic activity, signifying its role in substrate binding. More importantly, the Asp residue has been found to be strictly conserved among different HDAC isozymes, further emphasizing its importance in the substrate binding.
| + | |
| - | Based on the crystallographic studies, a mechanism of the HDAC8 catalyzed reaction has been proposed (Figure 1.5) [55]. The zinc ion plays a pivotal role in the entire catalytic process. It reduces the entropy by bringing the water and the acetyl-lysine substrate together to initiate the deacetylation reaction. Binding of the substrate to Zn2+ polarizes its carbonyl group thereby increasing its electrophilicity. In addition, the pKa of the Zn2+ bound water molecule is lowered, making it a stronger nucleophile. The deacetylation reaction starts with a nucleophilic attack of the catalytic water on the carbonyl carbon of the acetyl-lysine, producing a tetrahedral oxyanion intermediate that is stabilized via an electrostatic interaction with the Zn2+ ion as well as a hydrogen bonding with the hydroxyl group of Tyr 306. The collapse of the tetrahedral intermediate is mediated via the transfer of a proton from His 142, leading to the production of an acetate ion and a deacetylated lysine.
| + | |
| - | The release of the acetate ion generated during the HDAC8 catalysis is mediated via an acetate release channel (exit tunnel), which is located adjacent to the active site pocket. A similar channel has been observed in HDLP as shown in Figure 1.6 [58]. Hieder et al. have described the importance of a strictly conserved residue Arg 37 involved in the release of acetate ion through the exit pocket [61]. Mutation of Arg 37 to Ala reduces HDAC8 activity, suggesting that the product (acetate) release could be the rate limiting step in the HDAC8 catalyzed reaction. Additionally, Vannini et al. have proposed the involvement of Tyr 18, Tyr 20, and His 42 in the exchange of acetate with the bulk water [58]. These residues are located on the external surface of the HDAC8 exit tunnel. Recently, it has been demonstrated by Ruchi Khanna et al. that replacement of the above residues by mutagenesis with an Ala significantly reduces the kcat value of the HDAC8 catalyzed reaction with Fluoro-de-Lys substrate [unpublished data]. Although the residues proposed to be involved in the acetate release are strictly conserved among HDAC class I and II, no acetate release channel has been observed in the crystal structure of HDAC3, HDAC4, and HDAC7.
| + | |
| - | HDAC8 has several intriguing features which distinguish it from other HDAC isozymes. It lacks the 50-111 AAs segment extending beyond the C-terminal of the catalytic domain which is utilized to recruit other co-repressor/transcription factors [57]. It is likely that HDAC8 could have evolved to function in isolation. The crystallographic studies of HDAC8 with the structurally diverse hydroxamate inhibitors, namely, TSA, SAHA, M-334 and CRA-A, reveal an inherent malleability of its active site pocket, which is primarily due to the presence of the L1 loop (S30-K38) [57]. Moreover, the phosphorylation of Ser 39 by PKA has been reported to reduce the rate of the HDAC8 enzyme catalyzed reaction, presumably by slowing down the release of acetate through the release channel mediated via an electrostatic repulsion [58].
| + | |
| - | Recently, Cole at al. solved the crystal structure of HDAC8 bound with a depsipeptide inhibitor, Largazole (pdb 3RQD) [62]. The inhibitor is a pro-drug which produces a thiol moiety upon hydrolysis of the thioester linkage. The structure observed here was similar to the HDAC8-SAHA complex with the following salient differences. The thiolate ion serves as a monodentate ligand for the Zn2+ ion as opposed to SAHA, which is bidendate. The zinc ion coordination geometry is tetrahedral in case of HDAC8-largazole thiol complex, which is unique among all the crystal structures of HDAC isozymes known so far. Additionally, HDAC8 was required to undergo relatively larger conformational changes in the L1 and L2 loop region to accommodate the bulky and rigid inhibitor, largazole.
| + | |
| - | The crystal structures of HDAC8 containing different metal ions such as Co2+, Fe+2, and Zn2+ in the active site, have also been determined [63]. However, no significant difference in the HDAC8 structures was observed upon the substitution of the catalytic metal ion.
| + | |
| - | Danial P. Dowling et al. reported the crystal structure of HDAC8 in a monoclinic form (pdb 3F07), which was different from the form previously described in literature as orthorhombic [64]. Additionally, it was possible to obtain the crystal of the apo form of HDAC8, which was free from any ligand. A comparison of the ligand-bound (holoenzyme) vs. ligand -free (apoenzyme) forms of HDAC8 showed that the L2 loop becomes ordered upon binding to the ligand, APHA, 3-(1-methyl-4-phenylacetyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamide [64]
| + | |
| | | | |
| | == Disease == | | == Disease == |
| | | | |
| - | == Relevance == | + | HDAC8 is a part of the class I HDAC isozymes. This class is involved in expression of [http://en.wikipedia.org/wiki/P21 p21], a cyclin-dependent kinase inhibitor <ref>Blagosklonny, M. V. et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol. Cancer Ther. 1, 937–941 (2002).</ref>. The p21 proteins main job is to inhibit uncontrolled cell proliferation. A mutation in [http://proteopedia.org/wiki/index.php/P53 p53] gene has been reported in many cancer patients <ref name="Yan">Yan, W. et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene 32, 599–609 (2013).</ref>. HDAC8 regulates the expression of both the wild type and the mutant form of p53 <ref name="Yan" />.Inhibition of HDACs provides an anticancer effect <ref>Dokmanovic, M., Clarke, C. & Marks, P. A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol Cancer Res 5, 981–989 (2007).</ref>. However, a pan-HDAC inhibitor usually shows considerable side effects in a clinical setting. This is probably because multiple HDAC isozymes are involved in several vital cellular processes. Isozyme selective inhibitors are likely to have fewer side effects as compared to a pan-inhibitor. They induce growth inhibition, dedifferentiation, and even cell death in cancer cells <ref>Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5, 769–784 (2006).</ref>. It is widely known that a pan-HDAC inhibitor significantly affects the acetylation status of histones as well as several non-histone proteins, such as HSP90, p53 and others. The therapeutic potential of an HDAC8 selective activator for the treatment of various forms of cancers has recently emerged. In the above case the expression of the tumor suppressor protein (p53) is reportedly suppressed by HDAC8 <ref name="Yan" />. |
| | + | |
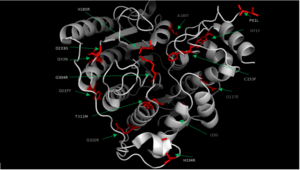
| | + | In some cases of [http://en.wikipedia.org/wiki/Cornelia_de_Lange_Syndrome Cornelia de Lange syndrome] (CLdS), the cohesion acetylation cycle is impaired due to mutations in HDAC8<ref name="Mut">Deardorff, M. A. et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489, 313–317 (2012).</ref>. The enzyme activity of all Class I HDACs has been found to be reduced in [http://en.wikipedia.org/wiki/Chronic_obstructive_pulmonary_disease Chronic Obstructive Pulmonary Disease] (COPD) <ref>Ito, K. et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967–1976 (2005).</ref>. Therapeutic potential of class I HDAC activators, specifically toward HDAC8, have not been well understood so far. However, there are several human diseases where an HDAC activator could be of great therapeutic benefits. In COPD and CLdS where the HDAC enzyme activity has been reported to be reduced, HDAC activators have a potential to lessen the disease conditions <ref name="Mut" />.[[Image:HDAC8_Mutations_Final.png|300px|left|thumb| '''Fig. 2''' Various mutations of HDAC8 found in CdLS patients.]] |
| | + | |
| | | | |
| | == Structural highlights == | | == Structural highlights == |
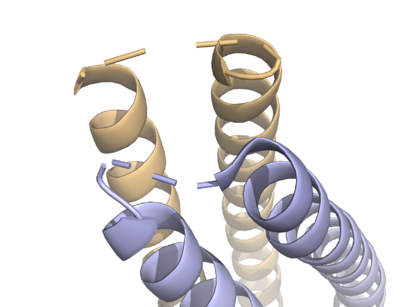
| | + | HDAC8 was the first among the HDAC isozymes whose crystal structure became available. The image shows the ribbon structure of <scene name='69/699997/Hdac8_saha/10'>HDAC8 bound with a canonical hydroxamate inhibitor, SAHA (Suberoylanilide Hydroxamic Acid)</scene>. HDAC8 contains a single α/β-deacetylase domain consisting of <scene name='69/699997/Hdac8_saha/11'>13 α-helices</scene> and an <scene name='69/699997/Hdac8_saha/13'>eight-stranded parallel β-sheet</scene>. [[Image:Loops.png|400px|left|thumb| '''Fig. 3''' The various loops associated with HDAC8.]]Multiple loops, which emanate from the protein core, play a significant role in maintaining the appropriate geometry of the catalytic pocket. The α/β-fold of the above kind was first observed in a metalloenzyme, [http://proteopedia.org/wiki/index.php/Arginase Arginase]. The active site of HDAC8 contains a tubular cavity leading to catalytic machinery at the end. The <scene name='69/699997/Hdac8_active_site_saha/18'>catalytic machinery is comprised of a Zn<sup>2+</sup> ion penta-coordinated</scene> with a square pyramidal geometry. The residues His 180, Asp 267 and Asp 178 occupy the three co-ordination sites, whereas the hydroxamate moiety of SAHA occupies the remaining two sites. In addition, the carbonyl oxygen of the hydroxamate moiety forms a hydrogen bond with Tyr 306. In the absence of any ligand (substrate/inhibitors), two water molecules are bound to the Zn<sup>2+</sup> ion. <scene name='69/699997/Dac8_active_site_haha/9'>The linker domain of the inhibitor interacts with the residues: Phe 152, Phe 208, His 180, Gly 151, and Met 274</scene>, which forms a <scene name='69/699997/Hdac8_tunnel/9'>hydrophobic tunnel</scene>. Notably, the above residues involved in the inhibitor/substrate binding, as well as the catalysis, have been found to be conserved during the course of evolution among class I HDACs. Notably, the carbonyl oxygen of the acetyl-lysine substrate forms a hydrogen bond with the hydroxyl moiety of Y306. |
| | + | Aside from the catalytic Zn<sup>2+</sup> ion, the enzyme activity of HDAC8 is dependent on the presence of the monovalent ion, K<sup>+</sup>/Na<sup>+</sup> <ref>Gantt, S. L., Joseph, C. G. & Fierke, C. A. Activation and Inhibition of Histone Deacetylase 8 by Monovalent Cations. J. Biol. Chem. 285, 6036–6043 (2010).</ref>. The crystal structure of HDAC8 shows the presence of two binding sites for K<sup>+</sup>/Na<sup>+</sup> <ref>Vannini, A. et al. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 15064–15069 (2004)</ref>. The <scene name='69/699997/K1_site/4'>first K+/Na+ binding site (K1)</scene> is located in the vicinity of the enzyme catalytic machinery, and it is hexacoordinated (octahedral geometry) with His 180 (carbonyl oxygen of the main chain), Asp 178 (oxygen atom of the main chain and side chain), Leu 200 (carbonyl oxygen of the main chain), and Ser 199 (O). Notably, His 180 and Asp 178 are the common residues coordinated with both the catalytic Zn<sup>2+</sup> as well as the K<sup>+</sup>/Na<sup>+</sup> ion. The <scene name='69/699997/K2_site/4'>second binding site for K+/Na+ ion (K2)</scene> is located near the surface. It is hexacoordinated (octahedral geometry) with F189, T192, V195, Y225 as well as two water molecule. |
| | + | |
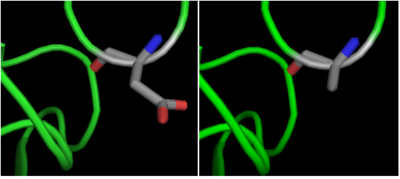
| | + | The crystal structure of <scene name='69/699997/Hdac8_binding_substrate/3'>HDAC8-substrate complex</scene> shows that aspartate residue <scene name='69/699997/Hdac8_d101/2'>(D101)</scene> is important in the substrate binding <ref>Vannini, A. et al. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports 8, 879–884 (2007).</ref>. The Asp 101 carboxylate moiety makes two consecutive hydrogen bonds with the backbone of the deacetylated peptide substrate. Mutation of Asp 101 to Ala inhibits HDAC8 activity. The Asp residue has been found to be strictly conserved among different HDAC isozymes. |
| | + | [[Image:D101.png|400px|left|thumb| '''Fig. 4''' Mutation of Asp 101 to Ala inhibits HDAC8 activity.]] |
| | + | |
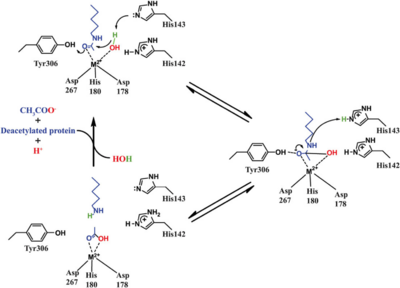
| | + | Based on the crystallographic studies, a mechanism of the HDAC8 catalyzed reaction has been proposed. |
| | + | [[Image:HDAC8_mechanism.png|400px|left|thumb| '''Fig. 5''' Catalytic mechanism of HDAC8.]] |
| | + | |
| | + | HDAC8 has a some structural features which make it different from other HDACs. It lacks the C-terminal sequence the others use to recruit cofactors <ref name="somoza">Somoza, J. R. et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12, 1325–1334 (2004).</ref>. The studies of HDAC8 with different hydroxamate inhibitors: <scene name='69/699997/Hdac8_tsa_bound/4'>TSA</scene>, <scene name='69/699997/Hdac8_binding_saha/6'>SAHA</scene> and <scene name='69/699997/Hdac8_binding_m-334/3'>M-334</scene> shows that there is a certain amount of flexibility in its active site pocket. This seems to be due to the presence of the <scene name='69/699997/L1_loop/4'>L1 loop (S30-K38)</scene> <ref name="somoza" />. |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | | | |
| - | <scene name='69/699997/Structure/1'>gay</scene> | |
| | | | |
| | </StructureSection> | | </StructureSection> |
| | == References == | | == References == |
| | <references/> | | <references/> |