Sandbox Reserved 1083
From Proteopedia
(Difference between revisions)
| (46 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{UniHelsinki_ProteinCourse_2015}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{UniHelsinki_ProteinCourse_2015}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | == | + | =='''AcrA'''== |
| - | <StructureSection load='2f1m' size='340' side='right' caption=' | + | <StructureSection load='2f1m' size='340' side='right' caption='AcrA tetramer' scene=''> |
| - | + | AcrA is a Multidrug efflux system protein. It belongs to resistance nodulation cell division (RND) family protein, which utilize electrochemical gradient to energize efflux of antibiotics and other compounds out of the bacterial cells <ref>PMID:11104814</ref>. RND system consists of large complexes of three essential proteins and work together as a multiprotein [http://en.wikipedia.org/wiki/Efflux_%28microbiology%29 efflux system]. Two most studied RND systems are ''Escherichia coli'' AcrA-AcrB-TolC and ''Pseudomonas aeruginosa'' MexA-MexB-OprM, which are known to efflux antibiotics, heavy metals, dyes, detergents, solvents, plus many other substrates <ref>PMID:15939505</ref>. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | AcrA is a Multidrug efflux system protein. It belongs to resistance nodulation cell division (RND) family protein, which utilize electrochemical gradient to energize efflux of antibiotics and other compounds out of the bacterial cells | + | |
== Stable core of AcrA == | == Stable core of AcrA == | ||
| - | Four molecules of AcrA (45-312 residues) in asymmetric unit of the crystal pack as an apparent <scene name='69/699996/Acra-dimer_of_dimers/1'>dimer of dimers</scene>. Each monomers are labeled as A (in | + | Four molecules of AcrA (45-312 residues) in asymmetric unit of the crystal pack as an apparent <scene name='69/699996/Acra-dimer_of_dimers/1'>dimer of dimers</scene>. Each monomers are labeled as A (in cyan), B (in orange), C (in green) and D (in yellow). A, B / C, D are related to one another by approximate <scene name='69/699996/Dyad_symmetry_final/1'>dyad symmetry</scene>. Each set of dimers are related to one another by approximate <scene name='69/699996/2_fold_axis_final/1'>2 fold axis</scene> <ref>PMID:16531241</ref>. |
| - | Each monomer is a sickle shaped molecule comprising three domains viz. β-barrel | + | Each <scene name='69/699996/Monomer_final/2'>monomer</scene> is a sickle shaped molecule comprising three domains viz. <scene name='69/699996/Beta_barrel_final/2'>β-barrel</scene>, <scene name='69/699996/Lipoyl_domain_final/2'>lipoyl domain</scene>, and coiled coil <scene name='69/699996/Hairpin_domain_final/1'>α-helical hairpin domain</scene>. β-barrel domain comprises six anti-parallel β-sheets and a short α-helix. Lipoyl domain is present in the central part of the AcrA monomer made up of <scene name='69/699996/Half_motifs/1'>two half motifs</scene> interrupted by an α-helical hairpin. Each half of the lipoyl motif is homologous to each other and consist of four β-strands in the form of a β-sandwich. The coiled coil domain consists of five [http://en.wikipedia.org/wiki/Heptad_repeat heptad repeats] per helix. Two α-helices are packed together as a canonical [http://en.wikipedia.org/wiki/Knobs_into_holes_packing knobs-into-holes] by hydrophobic side chains in the <scene name='69/699996/Knob_in_hole/4'>a and d positions</scene> of the heptad repeats <ref>PMID:10092468</ref> <ref>PMID:15507433</ref>. Crystal structure provide evidence for the flexibility of the hinge region between α-helical hairpin and lipoyl domain. The difference in hinge angle in case of B and C chain varies approximately by 15° overall and 21 Å at the loop located at the top of the hairpin. |
== Assembly within biological system == | == Assembly within biological system == | ||
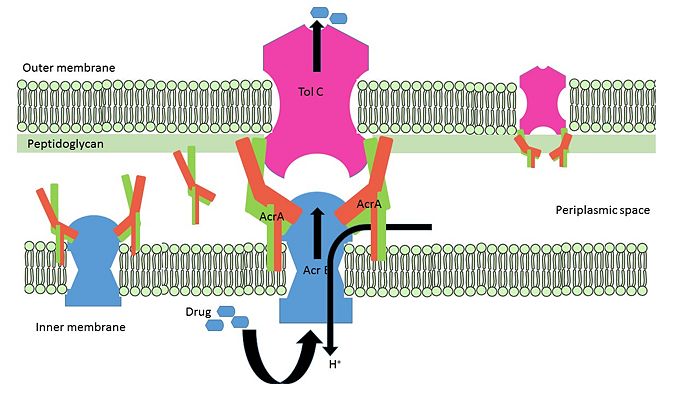
| - | AcrA is present within E. coli cells as a part of tripartite membrane associated efflux system along with AcrB and TolC. AcrA is present in the periplasmic space of cell with the proton antiporter AcrB in the inner-membrane and channel TolC in the outer membrane. It can it can remain free or form bipartite complexes with AcrB and TolC. The lipoyl and β-barrel domain of AcrA interact with AcrB, whereas the α-helical hairpin domain interact with TolC | + | [[Image: AcrA_AcrB_TolC_assembly.jpg|thumb|left|700px|Schematic representation of free AcrA; bipartite complex between AcrA-AcrB, AcrA-TolC; Tripartite complex between AcrAB-TolC.Figure modified from Qiang Ge et al, 2009]]. AcrA is present within ''E. coli'' cells as a part of tripartite membrane associated efflux system along with [http://www.uniprot.org/uniprot/P31224 AcrB] and [http://www.uniprot.org/uniprot/P02930 TolC]. AcrA is present in the periplasmic space of cell with the proton antiporter AcrB in the inner-membrane and channel TolC in the outer membrane. It can it can remain free or form bipartite complexes with AcrB and TolC. The lipoyl and β-barrel domain of AcrA interact with AcrB, whereas the α-helical hairpin domain interact with TolC <ref>PMID:19411330</ref>. |
| + | ArcA remains attached to the inner membrane via lipid acylation of Cys-25. N and C termini of AcrA form two <scene name='69/699996/Apposing_beta_final/1'>apposing β-strands</scene>, β1 (54-61) and β14 (292-297) lies proximal to inner membrane. A 28 flexible residues connects acylated Cys-25 with the β-barrel domain and allow the protein to reach the periplasmic top of AcrB <ref>PMID:12738864</ref>. A <scene name='69/699996/Short_alfa_helix_final/1'>short α-helix</scene> (222-230) located between β-10 (in red)(211-218) and β-11 (in blue) (242-247) closes off the end of the β-barrel near the C-terminus of AcrA fragment. Approximately 100 residues in the C-terminal are predicted to be important for AcrAB-TolC interaction. Structure has been resolved for homologous protein MexA from ''P. aeruginosa'', where similar conclusions on the role of N and C disordered domains in interaction with MexB has been predicted <ref>PMID:15117957</ref>. This segment contains 12 highly conserved amino acid residues viz. P309, V313, V332, R335, G352, L353, G356, D357, V359, V360, G363 and V373. G363 is found to be important for maintaining multidrug resistance property of the efflux system. Substitution of G363 by Cysteine (G363C), prevents appropriate assembly of the tripartite complex <ref>PMID:19411330</ref> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
AcrA
| |||||||||||
References
- ↑ Putman M, van Veen HW, Konings WN. Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev. 2000 Dec;64(4):672-93. PMID:11104814
- ↑ Kumar A, Schweizer HP. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev. 2005 Jul 29;57(10):1486-513. PMID:15939505 doi:http://dx.doi.org/10.1016/j.addr.2005.04.004
- ↑ Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. Conformational flexibility in the multidrug efflux system protein AcrA. Structure. 2006 Mar;14(3):577-87. PMID:16531241 doi:10.1016/j.str.2005.11.015
- ↑ Johnson JM, Church GM. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J Mol Biol. 1999 Apr 2;287(3):695-715. PMID:10092468 doi:http://dx.doi.org/10.1006/jmbi.1999.2630
- ↑ Akama H, Kanemaki M, Yoshimura M, Tsukihara T, Kashiwagi T, Yoneyama H, Narita S, Nakagawa A, Nakae T. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J Biol Chem. 2004 Dec 17;279(51):52816-9. Epub 2004 Oct 26. PMID:15507433 doi:10.1074/jbc.C400445200
- ↑ Ge Q, Yamada Y, Zgurskaya H. The C-terminal domain of AcrA is essential for the assembly and function of the multidrug efflux pump AcrAB-TolC. J Bacteriol. 2009 Jul;191(13):4365-71. doi: 10.1128/JB.00204-09. Epub 2009 May 1. PMID:19411330 doi:http://dx.doi.org/10.1128/JB.00204-09
- ↑ Yu EW, McDermott G, Zgurskaya HI, Nikaido H, Koshland DE Jr. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science. 2003 May 9;300(5621):976-80. PMID:12738864 doi:10.1126/science.1083137
- ↑ Akama H, Matsuura T, Kashiwagi S, Yoneyama H, Narita S, Tsukihara T, Nakagawa A, Nakae T. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J Biol Chem. 2004 Jun 18;279(25):25939-42. Epub 2004 Apr 26. PMID:15117957 doi:10.1074/jbc.C400164200
- ↑ Ge Q, Yamada Y, Zgurskaya H. The C-terminal domain of AcrA is essential for the assembly and function of the multidrug efflux pump AcrAB-TolC. J Bacteriol. 2009 Jul;191(13):4365-71. doi: 10.1128/JB.00204-09. Epub 2009 May 1. PMID:19411330 doi:http://dx.doi.org/10.1128/JB.00204-09