We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
JMS/Sandbox/trs
From Proteopedia
(Difference between revisions)
(New page: == Histidyl-tRNA Synthetase == '''Histidyl tRNA Synthetase''' (HisRS) is a 94kD <scene name='User:Jamie_Abbott/Sandbox2/Hisrsdimer/2'>homodimer</scene> that belongs to the class II of a...) |
|||
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
== Histidyl-tRNA Synthetase == | == Histidyl-tRNA Synthetase == | ||
| - | + | <StructureSection load='1KMM' size='500' side='right' caption='Structure of ''Escherichia coli'' Histidyl-tRNA Synthetase' scene=''> | |
| + | |||
'''Histidyl tRNA Synthetase''' (HisRS) is a 94kD <scene name='User:Jamie_Abbott/Sandbox2/Hisrsdimer/2'>homodimer</scene> that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). [http://www.pdb.org/pdb/101/motm.do?momID=16 Aminoacyl-tRNA synthetases] play a key role in protein synthesis and are classified as ligases. Aminoacyl tRNA synthetases use high energy ATP to attach a specific amino acid to its cognate tRNA<ref name="Eriani" />. This family of enzymes have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons<ref name="Eriani">PMID: 2203971</ref>. Class I and class II enzymes differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA<ref name="Eriani" />. Class I aaRS enzymes contain a conserved [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] catalytic domain and often monomeric. Furthermore, class I enzymes attach the activated amino acid to the 2'OH of the tRNA molecule during catalysis, which then migrates to the 3'OH. Class II enzymes have a <scene name='User:Jamie_Abbott/Sandbox2/Catalytic_domain/1'>catalytic domain</scene> composed of anti-parallel <scene name='User:Jamie_Abbott/Sandbox2/Catalytic_domainab/1'>β-sheets and α-helices</scene> (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be the <scene name='User:Jamie_Abbott/Sandbox2/Anticodon_binding_domain/1'>anticodon binding domain</scene>); class IIb, whose anticodon binding domain is located in the N-terminal region; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases <ref name="Cusack91">PMID: 1852601</ref>. | '''Histidyl tRNA Synthetase''' (HisRS) is a 94kD <scene name='User:Jamie_Abbott/Sandbox2/Hisrsdimer/2'>homodimer</scene> that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). [http://www.pdb.org/pdb/101/motm.do?momID=16 Aminoacyl-tRNA synthetases] play a key role in protein synthesis and are classified as ligases. Aminoacyl tRNA synthetases use high energy ATP to attach a specific amino acid to its cognate tRNA<ref name="Eriani" />. This family of enzymes have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons<ref name="Eriani">PMID: 2203971</ref>. Class I and class II enzymes differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA<ref name="Eriani" />. Class I aaRS enzymes contain a conserved [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] catalytic domain and often monomeric. Furthermore, class I enzymes attach the activated amino acid to the 2'OH of the tRNA molecule during catalysis, which then migrates to the 3'OH. Class II enzymes have a <scene name='User:Jamie_Abbott/Sandbox2/Catalytic_domain/1'>catalytic domain</scene> composed of anti-parallel <scene name='User:Jamie_Abbott/Sandbox2/Catalytic_domainab/1'>β-sheets and α-helices</scene> (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be the <scene name='User:Jamie_Abbott/Sandbox2/Anticodon_binding_domain/1'>anticodon binding domain</scene>); class IIb, whose anticodon binding domain is located in the N-terminal region; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases <ref name="Cusack91">PMID: 1852601</ref>. | ||
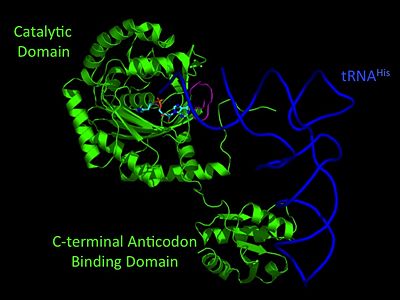
[http://www.brenda-enzymes.org/php/result_flat.php4?ecno=6.1.1.21 Histidyl-tRNA synthetases] catalyze the transfer of histidine to a histidinyl transfer RNA molecule (tRNAHis). As HisRS is a class II aaRS enzyme it attaches the amino acid histidine to the 3’OH of the terminal ribose of tRNA<ref name="aaRSbk" />. Histidine is often an important amino acid in the active site of other enzymes and is unique as it can behave as either an acid or a base. The overall secondary structure of a HisRS monomer consist of 20% beta sheets and 37% helical character. It is structurally classified by CATH as an α-β layered sandwich. The CATH hierarchy of structural classification is based on: Class, Architecture, Topology, Homologous superfamily. Each <scene name='User:Jamie_Abbott/Sandbox2/Hisrsdimer_to_monomer/2'>monomer</scene> of HisRS contains a N-terminal catalytic domain, C-terminal anticodon binding domain, a <scene name='User:Jamie_Abbott/Sandbox2/Motif_i/3'>Motif I</scene>, II, and III, an insertion domain, a HisA and HisB loop, as well as an insertion domain. These structural elements are essential in assisting the two step mechanism carried out to aminoacylate tRNA<sup>His</sup> with high fidelity. | [http://www.brenda-enzymes.org/php/result_flat.php4?ecno=6.1.1.21 Histidyl-tRNA synthetases] catalyze the transfer of histidine to a histidinyl transfer RNA molecule (tRNAHis). As HisRS is a class II aaRS enzyme it attaches the amino acid histidine to the 3’OH of the terminal ribose of tRNA<ref name="aaRSbk" />. Histidine is often an important amino acid in the active site of other enzymes and is unique as it can behave as either an acid or a base. The overall secondary structure of a HisRS monomer consist of 20% beta sheets and 37% helical character. It is structurally classified by CATH as an α-β layered sandwich. The CATH hierarchy of structural classification is based on: Class, Architecture, Topology, Homologous superfamily. Each <scene name='User:Jamie_Abbott/Sandbox2/Hisrsdimer_to_monomer/2'>monomer</scene> of HisRS contains a N-terminal catalytic domain, C-terminal anticodon binding domain, a <scene name='User:Jamie_Abbott/Sandbox2/Motif_i/3'>Motif I</scene>, II, and III, an insertion domain, a HisA and HisB loop, as well as an insertion domain. These structural elements are essential in assisting the two step mechanism carried out to aminoacylate tRNA<sup>His</sup> with high fidelity. | ||
| + | |||
| + | |||
== Substrate Specificity == | == Substrate Specificity == | ||
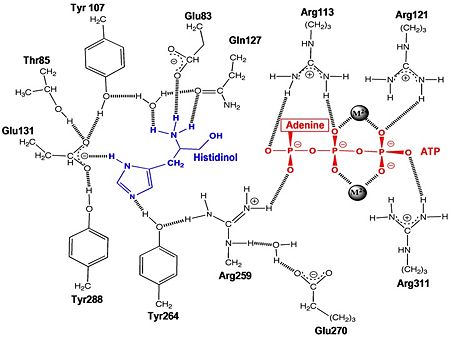
| - | + | The first crystal structure solved for histidyl tRNA-synthetase was of E.coli. To date structures of E.coli HisRS complexed with ATP, AMP, histidine, competitive inhibitor (histidinol), histidyl-adenylate, and 5’-O-[(L-histidinylamino)sulfonyl] adenosine have been extensively explored. Many residues involved in substrate specificity and binding were identified. Not only have residues important for substrate binding been identified but also residues essential for catalysis. | |
| Line 27: | Line 30: | ||
'''A Role for Coordinated Metal Ions''' | '''A Role for Coordinated Metal Ions''' | ||
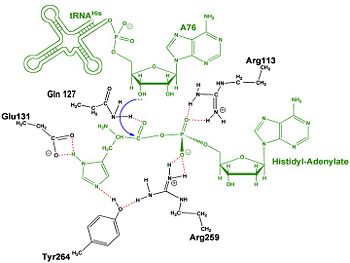
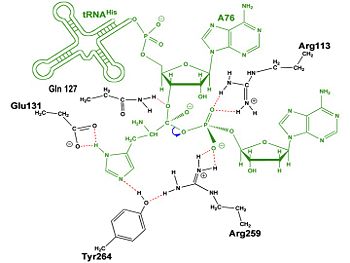
| - | Thus far, residues of HisRS have been described for the binding of substrates histidine and ATP. However, HisRS also requires two magnesium ions to carry out catalysis. Most importantly, the β and γ phosphates of ATP are neutralized by two coordinated magnesium ions that are positioned by water molecules and conserved Glu115. Weak electron density, consistent with a bound Mg<sup>2+</sup> ion, was observed in an electron density map for the HisRS:histidinol:ATP complex <ref name="Arnez97" />. This particular Mg<sup>2+</sup> ion coordinates the β and γ phosphates of ATP. Arnez et al. further defined the locations of magnesium ions by taking a crystal of HisRS:histidinol:ATP complex and soaking it in manganese(II) chloride MnCl2. These data showed that two Mn<sup>2+</sup> ions coordinate the β and γ phosphates of ATP. Furthermore, interatomic distances between the Mn<sup>2+</sup> principal ion and the β phosphate oxygen is approximately 0.5 Å, which would be expected to contribute to catalysis by weakening the bond between the α and β phosphates of ATP. In similar manganese soaking experiments with another classIIa aaRS, SerRS, the principal metal ion was shown to coordinate the α and β phosphates<ref name="belrhali">PMID: 7613865</ref>. The functional role for the metal ion coordination between the α and β phosphates for SerRS is a metal-catalyzed mechanism for the adenylation reaction. Interestingly, Arg259 in the HisRS:ATP complex resides in the position occupied by the metal catalyst Mg<sup>2+</sup>, in classIIa SerRS. Arg259 and Arg113 serving in place of a Mg<sup>2+</sup> ion is unique to HisRS compared to other classII aaRS. Other classII aaRS enzymes have conserved carboxylate groups to assist coordination of metal ions to carry out catalysis, while HisRS has in place residues Glu270 and Thr281 that have poor geometry for metal coordination but participate in the arginine salt bridge switch<ref name="Arnez97" />. | + | Thus far, residues of HisRS have been described for the binding of substrates histidine and ATP. However, HisRS also requires two magnesium ions to carry out catalysis. Most importantly, the β and γ phosphates of ATP are neutralized by two coordinated magnesium ions that are positioned by water molecules and conserved Glu115. Weak electron density, consistent with a bound Mg<sup>2+</sup> ion, was observed in an electron density map for the HisRS:histidinol:ATP complex <ref name="Arnez97" />. This particular Mg<sup>2+</sup> ion coordinates the β and γ phosphates of ATP. Arnez et al. further defined the locations of magnesium ions by taking a crystal of HisRS:histidinol:ATP complex and soaking it in manganese(II) chloride MnCl2. These data showed that two Mn<sup>2+</sup> ions coordinate the β and γ phosphates of ATP. Furthermore, interatomic distances between the Mn<sup>2+</sup> principal ion and the β phosphate oxygen is approximately 0.5 Å, which would be expected to contribute to catalysis by weakening the bond between the α and β phosphates of ATP. In similar manganese soaking experiments with another classIIa aaRS, SerRS, the principal metal ion was shown to coordinate the α and β phosphates<ref name="belrhali">PMID: 7613865</ref>. The functional role for the metal ion coordination between the α and β phosphates for SerRS is a metal-catalyzed mechanism for the adenylation reaction. Interestingly, Arg259 in the HisRS:ATP complex resides in the position occupied by the metal catalyst Mg<sup>2+</sup>, in classIIa SerRS. Arg259 and Arg113 serving in place of a Mg<sup>2+</sup> ion is unique to HisRS compared to other classII aaRS. Other classII aaRS enzymes have conserved carboxylate groups to assist coordination of metal ions to carry out catalysis, while HisRS has in place residues Glu270 and Thr281 that have poor geometry for metal coordination but participate in the arginine salt bridge switch<ref name="Arnez97" />.(see figure below) |
| + | |||
| + | |||
---- | ---- | ||
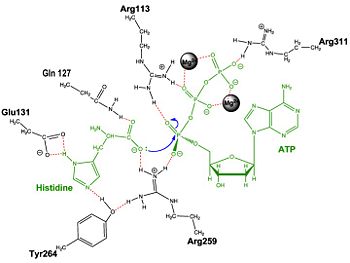
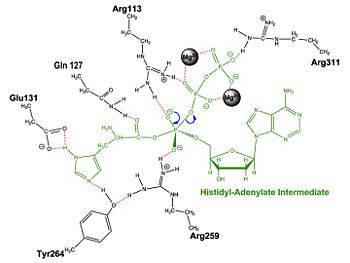
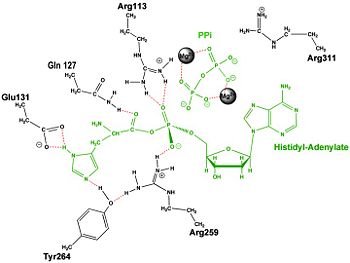
== Mechanism of the Adenylation Reaction== | == Mechanism of the Adenylation Reaction== | ||
| - | + | below | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
---- | ---- | ||
| - | + | ||
| + | |||
| + | |||
---- | ---- | ||
=== Electrophilic Catalysis === | === Electrophilic Catalysis === | ||
| Line 46: | Line 50: | ||
A comparison of <scene name='User:Jamie_Abbott/Sandbox2/Glu270_distance/1'>Glu270</scene> in the HisRS:histidinol and the HisRS:adenylate complexes provides further structural information into how Arg259 may serve a role in catalysis. In the HisRS:histidinol complex a water-mediated interaction exists between Glu270 and εN of Arg259. However, in the HisRS:adenylate complex Glu270 moves to form a salt bridge with the guanidinium group excluding the water molecule. This movement serves as a salt bridge switch that may weaken the ionic interaction between Arg259 and the α-phosphate<ref name="Arnez97" /> and stabilize adenylate formation in the active site. Also, Arg113 as well as Arg259 are arranged to interact with <scene name='User:Jamie_Abbott/Sandbox2/His_r259_r113/3'>α-phosphate</scene> of the histidyl-adenylate intermediate and stabilizes negative charge developed on the non-bridging oxygens α-phosphate during the transition state <ref name="aaRSbk" />. Evidence for Arg259 as critical residue in catalysis is further supported by mutational studies where a two or three log decrease in activity is observed when Arg259 is substituted with histidine <ref name="Arnez97" /> or other amino acids<ref name="arnez1">PMID: 9266856</ref>. Utilizing Arg259 for catalysis is unique to HisRS as other class II aaRS enzymes, AspRS<ref>PMID: 7966328</ref> and SerRS<ref name="belrhali" />, use a divalent magnesium metal ion to coordinate the α-phosphate of ATP and serve as an electrophilic catalyst. | A comparison of <scene name='User:Jamie_Abbott/Sandbox2/Glu270_distance/1'>Glu270</scene> in the HisRS:histidinol and the HisRS:adenylate complexes provides further structural information into how Arg259 may serve a role in catalysis. In the HisRS:histidinol complex a water-mediated interaction exists between Glu270 and εN of Arg259. However, in the HisRS:adenylate complex Glu270 moves to form a salt bridge with the guanidinium group excluding the water molecule. This movement serves as a salt bridge switch that may weaken the ionic interaction between Arg259 and the α-phosphate<ref name="Arnez97" /> and stabilize adenylate formation in the active site. Also, Arg113 as well as Arg259 are arranged to interact with <scene name='User:Jamie_Abbott/Sandbox2/His_r259_r113/3'>α-phosphate</scene> of the histidyl-adenylate intermediate and stabilizes negative charge developed on the non-bridging oxygens α-phosphate during the transition state <ref name="aaRSbk" />. Evidence for Arg259 as critical residue in catalysis is further supported by mutational studies where a two or three log decrease in activity is observed when Arg259 is substituted with histidine <ref name="Arnez97" /> or other amino acids<ref name="arnez1">PMID: 9266856</ref>. Utilizing Arg259 for catalysis is unique to HisRS as other class II aaRS enzymes, AspRS<ref>PMID: 7966328</ref> and SerRS<ref name="belrhali" />, use a divalent magnesium metal ion to coordinate the α-phosphate of ATP and serve as an electrophilic catalyst. | ||
| + | |||
== Mechanism of the Aminoacylation Reaction== | == Mechanism of the Aminoacylation Reaction== | ||
| - | + | below | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[Image:ProRproS.jpg|thumb|left|upright=1.0|400px|'''Substrate assisted mechanism catalyzed by histidyl-tRNA synthetase''' proposed by Francklyn et al.<ref name="GUTH05" />]] | ||
=== Substrate Assisted Catalysis === | === Substrate Assisted Catalysis === | ||
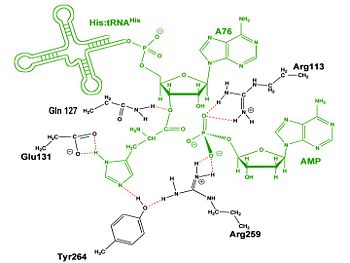
The second reaction carried out by HisRS, <scene name='User:Jamie_Abbott/Sandbox2/Adenylate_binding_residues/1'>aminoacylation</scene>, requires the decomposition of a mixed anhydride (the <scene name='User:Jamie_Abbott/Sandbox2/Histidyl-adenylate/1'>histidyl-adenylate</scene>) to form an aminoacyl ester on the 3’OH of tRNA<sup>His</sup>. It was initially hypothesized that Glu83, acting as a general base, would improve the rate of this reaction. However, while Glu83 is in a favorable position in the active site to function as a base it is also situated to neutralize the α-amino group of the histidine substrate. Mutational analysis of Glu83<ref name ="GUTH05">PMID: 15751955</ref> suggests that it does not act as a base but forms a salt bridge with the α-amino group of histidine, neutralizing the charge, and satisfying a critical electrostatic interaction. | The second reaction carried out by HisRS, <scene name='User:Jamie_Abbott/Sandbox2/Adenylate_binding_residues/1'>aminoacylation</scene>, requires the decomposition of a mixed anhydride (the <scene name='User:Jamie_Abbott/Sandbox2/Histidyl-adenylate/1'>histidyl-adenylate</scene>) to form an aminoacyl ester on the 3’OH of tRNA<sup>His</sup>. It was initially hypothesized that Glu83, acting as a general base, would improve the rate of this reaction. However, while Glu83 is in a favorable position in the active site to function as a base it is also situated to neutralize the α-amino group of the histidine substrate. Mutational analysis of Glu83<ref name ="GUTH05">PMID: 15751955</ref> suggests that it does not act as a base but forms a salt bridge with the α-amino group of histidine, neutralizing the charge, and satisfying a critical electrostatic interaction. | ||
| Line 67: | Line 67: | ||
== Histidinyl tRNA Recognition == | == Histidinyl tRNA Recognition == | ||
| - | + | below | |
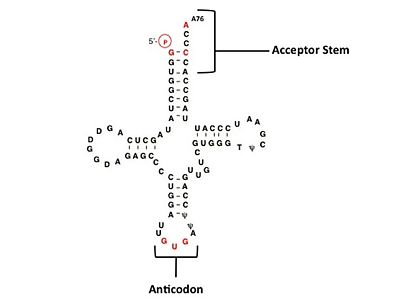
The accuracy of protein synthesis is dependent upon the ability of aminoacyl-tRNA synthetases to specifically recognize the cognate tRNA and attach the appropriate amino acid. The identity nucleotides that define tRNA isoacceptor systems are primarily concentrated in the anticodon region and acceptor stems of [http://proteopedia.org/wiki/index.php/TRNA tRNA molecules]. These nucleotides provide functional groups that can be accessed by specific amino acid side chains of the aaRS enzymes<ref>PMID: 8422978</ref>,<ref>PMID: 1857417</ref>. Also, tRNA identity and recognition can also emerge from the presence of modified bases such those commonly seen in the D-arm of tRNA molecules, dihydrouridine, or less common modification such as C5-methylation of uracil<ref>PMID: 3054566</ref>. Key identity elements on ''E. coli'' histidine tRNA include the 5’ phosphate, G-1:C73 base pair in the acceptor stem and the GUG anticodon. Mutations of these identity elements diminishes aminoacylation ''in vitro''<ref>PMID: 10747795</ref> <ref>PMID: 2678006</ref> <ref>PMID: 8643360</ref>. Residues thought to be involved in the <scene name='User:Jamie_Abbott/Sandbox2/Monomer-trna_recognition/1'>recognition </scene>of these identity elements include: Arg123, Arg116, and Gln118. Substitution in Arg123 as a putative contact to the 5’phosphate, produced a 200 fold decrease in aminoacyl-transfer<ref name ="GUTH07">PMID: 17317626</ref>. Similar kinetic defects in aminoacyl-transfer were also observed for Arg116 and Gln118 <ref name="GUTH07" />. | The accuracy of protein synthesis is dependent upon the ability of aminoacyl-tRNA synthetases to specifically recognize the cognate tRNA and attach the appropriate amino acid. The identity nucleotides that define tRNA isoacceptor systems are primarily concentrated in the anticodon region and acceptor stems of [http://proteopedia.org/wiki/index.php/TRNA tRNA molecules]. These nucleotides provide functional groups that can be accessed by specific amino acid side chains of the aaRS enzymes<ref>PMID: 8422978</ref>,<ref>PMID: 1857417</ref>. Also, tRNA identity and recognition can also emerge from the presence of modified bases such those commonly seen in the D-arm of tRNA molecules, dihydrouridine, or less common modification such as C5-methylation of uracil<ref>PMID: 3054566</ref>. Key identity elements on ''E. coli'' histidine tRNA include the 5’ phosphate, G-1:C73 base pair in the acceptor stem and the GUG anticodon. Mutations of these identity elements diminishes aminoacylation ''in vitro''<ref>PMID: 10747795</ref> <ref>PMID: 2678006</ref> <ref>PMID: 8643360</ref>. Residues thought to be involved in the <scene name='User:Jamie_Abbott/Sandbox2/Monomer-trna_recognition/1'>recognition </scene>of these identity elements include: Arg123, Arg116, and Gln118. Substitution in Arg123 as a putative contact to the 5’phosphate, produced a 200 fold decrease in aminoacyl-transfer<ref name ="GUTH07">PMID: 17317626</ref>. Similar kinetic defects in aminoacyl-transfer were also observed for Arg116 and Gln118 <ref name="GUTH07" />. | ||
| - | + | (see figure below) | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
| + | |||
=== Structural Homology === | === Structural Homology === | ||
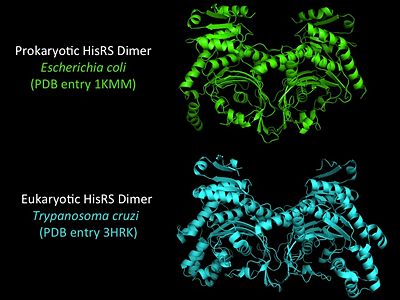
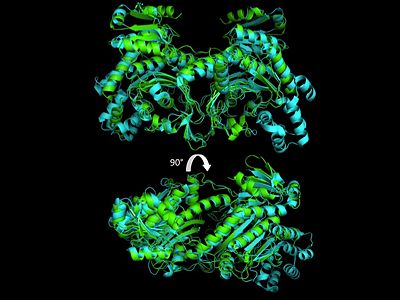
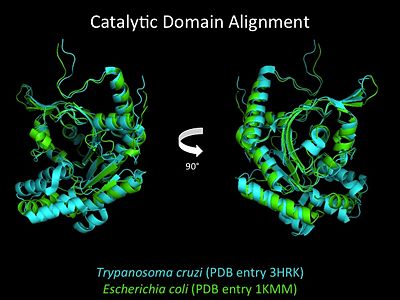
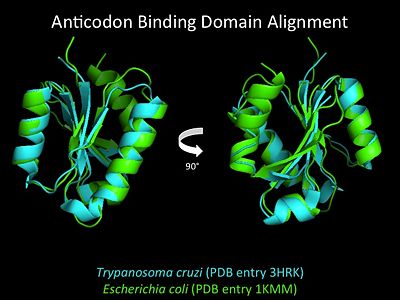
| Line 80: | Line 81: | ||
More recently a crystal structures for eukaryotic ''Trypanosomal brucei'' and ''Trypanosomal cruzi'' histidyl-tRNA synthetase was solved<ref>PMID: 20132829</ref>. While both the human and trypanosomal HisRS sequences are part of the same eukaryotic branch on the HisRS phylogenetic tree<ref>PMID: 17182897</ref> there is less than 30% sequence identity shared between them. Similarly, the sequence identity between trypanosoaml HisRS and bacterial HisRS is less than 30% <ref>PMID: 20132829</ref>. Furthermore, many higher eukaryotes, more specifically mammals, have separate cytosolic and mitochondrial HisRS enzymes. The human HisRS genes, which arose from inverted gene duplication<ref>PMID: 12056811</ref><ref>PMID: 7755634</ref>, HARS and HARS2 encode for the cytosolic and mitochondrial HisRS enzymes respectively. The gene products of HARS and HARS2 share approximately 79% sequence identity. | More recently a crystal structures for eukaryotic ''Trypanosomal brucei'' and ''Trypanosomal cruzi'' histidyl-tRNA synthetase was solved<ref>PMID: 20132829</ref>. While both the human and trypanosomal HisRS sequences are part of the same eukaryotic branch on the HisRS phylogenetic tree<ref>PMID: 17182897</ref> there is less than 30% sequence identity shared between them. Similarly, the sequence identity between trypanosoaml HisRS and bacterial HisRS is less than 30% <ref>PMID: 20132829</ref>. Furthermore, many higher eukaryotes, more specifically mammals, have separate cytosolic and mitochondrial HisRS enzymes. The human HisRS genes, which arose from inverted gene duplication<ref>PMID: 12056811</ref><ref>PMID: 7755634</ref>, HARS and HARS2 encode for the cytosolic and mitochondrial HisRS enzymes respectively. The gene products of HARS and HARS2 share approximately 79% sequence identity. | ||
| + | |||
| + | </StructureSection> | ||
| + | |||
| + | |||
| + | ==Substrate Specificity== | ||
| + | [[Image:HisRShisolATP.jpg |thumb|left|upright=3.0|450px|'''Active Site Residues''']] | ||
| + | <br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /> | ||
| + | ==Mechanism of the Adenylation Reaction== | ||
| + | {| | ||
| + | | [[Image:Adnrxn1.jpg|center|350px|'''Adenylation reaction catalyzed by HisRS.''']] | ||
| + | | [[Image:Adnrxn2.jpg|center|350px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
| + | | [[Image:Adnrxn3.jpg|center|350px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
| + | |} | ||
| + | <br /> | ||
| + | == Mechanism of the Aminoacylation Reaction== | ||
| + | {| | ||
| + | | [[Image:Amarxn1.jpg|center|350px|'''Adenylation reaction catalyzed by HisRS.''']] | ||
| + | | [[Image:Amarxn2.jpg|center|350px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
| + | | [[Image:Amarxn3.jpg|center|350px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
| + | |} | ||
| + | <br /> | ||
| + | [[Image:ProRproS.jpg|thumb|left|upright=1.0|400px|'''Substrate assisted mechanism catalyzed by histidyl-tRNA synthetase''' proposed by Francklyn et al.<ref name="GUTH05" />]] | ||
| + | <br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /> | ||
| + | == Histidinyl tRNA Recognition == | ||
| + | [[Image:TRNAHis2.jpg |thumb|left|upright=2.0|400px|'''Clover leaf structure of histidinyl tRNA from E.coli''' key recognition elements are shown in red]] | ||
| + | <br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /> | ||
| + | [[Image:HisRS+tRNAHis.jpg|thumb|left|upright=2.0|400px|'''Model of HisRS-tRNAHis Complex''' predicted by homology modeling with AspRS-tRNAAsp crystal structure<ref>PMID: 14744140</ref>]] | ||
| + | <br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /> | ||
| + | == put title here == | ||
{| | {| | ||
| [[Image:HisRSeukaryotic+prokaryotic.jpg|center|400px|'''Adenylation reaction catalyzed by HisRS.''']] | | [[Image:HisRSeukaryotic+prokaryotic.jpg|center|400px|'''Adenylation reaction catalyzed by HisRS.''']] | ||
| [[Image:HisRSP+Edimeralign.jpg|center|400px|'''Aminoacylation reaction catalyzed by HisRS.''']] | | [[Image:HisRSP+Edimeralign.jpg|center|400px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
|} | |} | ||
| + | <br /> | ||
| + | |||
{| | {| | ||
| [[Image:HisRScatalyticdomalign.jpg|center|400px|'''Adenylation reaction catalyzed by HisRS.''']] | | [[Image:HisRScatalyticdomalign.jpg|center|400px|'''Adenylation reaction catalyzed by HisRS.''']] | ||
| [[Image:HisRSacbinddomalign.jpg |center|400px|'''Aminoacylation reaction catalyzed by HisRS.''']] | | [[Image:HisRSacbinddomalign.jpg |center|400px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
|} | |} | ||
| - | + | <br /> | |
== 3D Structures of Histidyl-tRNA Synthetase == | == 3D Structures of Histidyl-tRNA Synthetase == | ||
Current revision
Contents |
Histidyl-tRNA Synthetase
| |||||||||||
Substrate Specificity
Mechanism of the Adenylation Reaction
Mechanism of the Aminoacylation Reaction
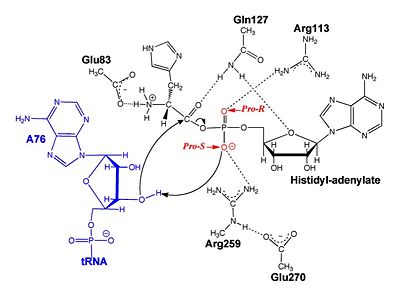
Substrate assisted mechanism catalyzed by histidyl-tRNA synthetase proposed by Francklyn et al.[11]
Histidinyl tRNA Recognition
Model of HisRS-tRNAHis Complex predicted by homology modeling with AspRS-tRNAAsp crystal structure[25]
put title here
3D Structures of Histidyl-tRNA Synthetase
Bacteria
Eukaryota
Archara
References
- ↑ 1.0 1.1 1.2 Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203-6. PMID:2203971 doi:http://dx.doi.org/10.1038/347203a0
- ↑ Cusack S, Hartlein M, Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489-98. PMID:1852601
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Francklyn, C., and Arnez, J.G. (2004) in Aminoacyl-tRNA Synthetases (Ibba, M.,Francklyn, C.,Cusack, S.. Eds.) Landes Publishing, Austin, TX
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Arnez JG, Augustine JG, Moras D, Francklyn CS. The first step of aminoacylation at the atomic level in histidyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7144-9. PMID:9207058
- ↑ Arnez JG, Harris DC, Mitschler A, Rees B, Francklyn CS, Moras D. Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate. EMBO J. 1995 Sep 1;14(17):4143-55. PMID:7556055
- ↑ Arnez JG, Moras D. Structural and functional considerations of the aminoacylation reaction. Trends Biochem Sci. 1997 Jun;22(6):211-6. PMID:9204708
- ↑ 7.0 7.1 Belrhali H, Yaremchuk A, Tukalo M, Berthet-Colominas C, Rasmussen B, Bosecke P, Diat O, Cusack S. The structural basis for seryl-adenylate and Ap4A synthesis by seryl-tRNA synthetase. Structure. 1995 Apr 15;3(4):341-52. PMID:7613865
- ↑ Arnez JG, Flanagan K, Moras D, Simonson T. Engineering an Mg2+ site to replace a structurally conserved arginine in the catalytic center of histidyl-tRNA synthetase by computer experiments. Proteins. 1998 Aug 15;32(3):362-80. PMID:9715912
- ↑ Ruhlmann A, Cramer F, Englisch U. Isolation and analysis of mutated histidyl-tRNA synthetases from Escherichia coli. Biochem Biophys Res Commun. 1997 Aug 8;237(1):192-201. PMID:9266856 doi:10.1006/bbrc.1997.7108
- ↑ Poterszman A, Delarue M, Thierry JC, Moras D. Synthesis and recognition of aspartyl-adenylate by Thermus thermophilus aspartyl-tRNA synthetase. J Mol Biol. 1994 Nov 25;244(2):158-67. PMID:7966328 doi:http://dx.doi.org/10.1006/jmbi.1994.1716
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Guth E, Connolly SH, Bovee M, Francklyn CS. A substrate-assisted concerted mechanism for aminoacylation by a class II aminoacyl-tRNA synthetase. Biochemistry. 2005 Mar 15;44(10):3785-94. PMID:15751955 doi:10.1021/bi047923h
- ↑ 12.0 12.1 12.2 12.3 Guth EC, Francklyn CS. Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Mol Cell. 2007 Feb 23;25(4):531-42. PMID:17317626 doi:10.1016/j.molcel.2007.01.015
- ↑ Moulinier L, Eiler S, Eriani G, Gangloff J, Thierry JC, Gabriel K, McClain WH, Moras D. The structure of an AspRS-tRNA(Asp) complex reveals a tRNA-dependent control mechanism. EMBO J. 2001 Sep 17;20(18):5290-301. PMID:11566892 doi:http://dx.doi.org/10.1093/emboj/20.18.5290
- ↑ Cavarelli J, Moras D. Recognition of tRNAs by aminoacyl-tRNA synthetases. FASEB J. 1993 Jan;7(1):79-86. PMID:8422978
- ↑ Rould MA, Perona JJ, Steitz TA. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature. 1991 Jul 18;352(6332):213-8. PMID:1857417 doi:http://dx.doi.org/10.1038/352213a0
- ↑ Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988 Nov 10;336(6195):179-81. PMID:3054566 doi:http://dx.doi.org/10.1038/336179a0
- ↑ Fromant M, Plateau P, Blanquet S. Function of the extra 5'-phosphate carried by histidine tRNA. Biochemistry. 2000 Apr 11;39(14):4062-7. PMID:10747795
- ↑ Himeno H, Hasegawa T, Ueda T, Watanabe K, Miura K, Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNA(His) in aminoacylation. Nucleic Acids Res. 1989 Oct 11;17(19):7855-63. PMID:2678006
- ↑ Yan W, Francklyn C. tRNA selection by a class II aminoacyl-tRNA synthetase: the role of accessory domains and inter-domain communication in RNA recognition. Nucleic Acids Symp Ser. 1995;(33):167-9. PMID:8643360
- ↑ Merritt EA, Arakaki TL, Gillespie JR, Larson ET, Kelley A, Mueller N, Napuli AJ, Kim J, Zhang L, Verlinde CL, Fan E, Zucker F, Buckner FS, van Voorhis WC, Hol WG. Crystal structures of trypanosomal histidyl-tRNA synthetase illuminate differences between eukaryotic and prokaryotic homologs. J Mol Biol. 2010 Mar 26;397(2):481-94. Epub 2010 Feb 2. PMID:20132829 doi:10.1016/j.jmb.2010.01.051
- ↑ Brindefalk B, Viklund J, Larsson D, Thollesson M, Andersson SG. Origin and evolution of the mitochondrial aminoacyl-tRNA synthetases. Mol Biol Evol. 2007 Mar;24(3):743-56. Epub 2006 Dec 20. PMID:17182897 doi:10.1093/molbev/msl202
- ↑ Merritt EA, Arakaki TL, Gillespie JR, Larson ET, Kelley A, Mueller N, Napuli AJ, Kim J, Zhang L, Verlinde CL, Fan E, Zucker F, Buckner FS, van Voorhis WC, Hol WG. Crystal structures of trypanosomal histidyl-tRNA synthetase illuminate differences between eukaryotic and prokaryotic homologs. J Mol Biol. 2010 Mar 26;397(2):481-94. Epub 2010 Feb 2. PMID:20132829 doi:10.1016/j.jmb.2010.01.051
- ↑ O'Hanlon TP, Miller FW. Genomic organization, transcriptional mapping, and evolutionary implications of the human bi-directional histidyl-tRNA synthetase locus (HARS/HARSL). Biochem Biophys Res Commun. 2002 Jun 14;294(3):609-14. PMID:12056811 doi:10.1016/S0006-291X(02)00525-9
- ↑ O'Hanlon TP, Raben N, Miller FW. A novel gene oriented in a head-to-head configuration with the human histidyl-tRNA synthetase (HRS) gene encodes an mRNA that predicts a polypeptide homologous to HRS. Biochem Biophys Res Commun. 1995 May 16;210(2):556-66. PMID:7755634
- ↑ Connolly SA, Rosen AE, Musier-Forsyth K, Francklyn CS. G-1:C73 recognition by an arginine cluster in the active site of Escherichia coli histidyl-tRNA synthetase. Biochemistry. 2004 Feb 3;43(4):962-9. PMID:14744140 doi:10.1021/bi035708f