Gluten
From Proteopedia
(Difference between revisions)
| (22 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
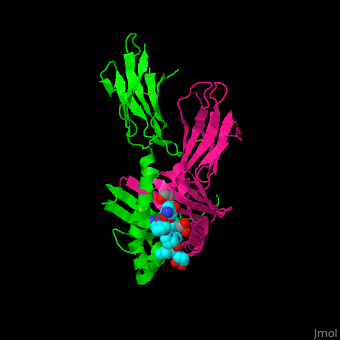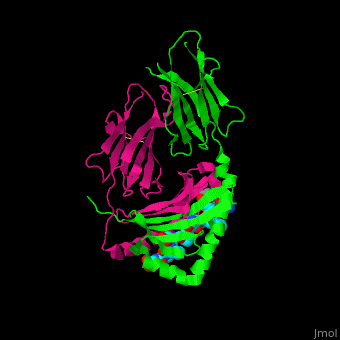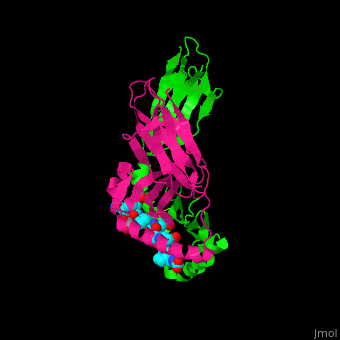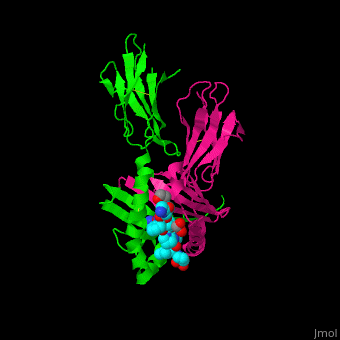
| - | == | + | <StructureSection load='' size='450' side='right' scene ='71/719201/Cv/1' caption = 'Synthetic gliadin peptide (cyan) complex with HLA class II histocompatibility antigen (α chain in magenta, β chain in green) and ethylene glycol (PDB code [[1s9v]])'> |
| - | + | __TOC__ | |
| + | == Introduction == | ||
| - | + | The protein [https://en.wikipedia.org/wiki/Gluten gluten] is found in wheat and grains such as rye and barley. Gluten is also involved with inducing an inflammatory response in individuals with [https://en.wikipedia.org/wiki/Coeliac_disease celiac disease]. Individuals who have the disease cannot digest gluten due to the structure of the protein, which will damage the small intestine. If an individual with '''celiac disease''' ingests foods containing gluten, the immune system responds by damaging the villi, which are fingerlike projections lining the small intestine. This immune response reduces the body’s ability to absorb nutrients that pass through the small intestine and into the bloodstream. As a result of the damaged villi, people with celiac disease can become malnourished. Although celiac disease is genetic, the research reviewed for this study placed emphasis on how the protein triggers an immune response in the gastrointestinal tract of affected individuals. | |
| - | + | '''Gluten''' is a protein complex comprised of 2 components: '''gliadin''' (the water-soluble component) and '''glutenin''' (the water-isoluble component). Gliadins, for those with celiac disease, are the principle toxic component of gluten and are composed of proline and glutamine-rich peptide sequences. The peptides enter the circulatory system and come into contact with [https://en.wikipedia.org/wiki/Lymphocyte lymphocytes] and [https://en.wikipedia.org/wiki/T_cell T-cells], resulting in the release of cytokines. The [https://en.wikipedia.org/wiki/Cytokine cytokines] interact with the villi of the small intestine and damage them, disabling the body from nutrient absorption. The symptoms can include abdominal pain, weight loss, fatigue, and many other symptoms associated with malnutrition. As of now, the only treatment for celiac disease is the total exclusion of gluten from the person’s diet.<ref>Celiac Disease: MedlinePlus. Retrieved October 27, 2015, from https://www.nlm.nih.gov/medlineplus/celiacdisease.html</ref><ref>Celiac Disease. Retrieved October 27, 2015, from http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/celiac-disease/Pages/facts.aspx</ref><ref>Go to Science. Retrieved October 27, 2015, from http://www.sciencemag.org/content/297/5590/2275.full</ref> | |
| + | <scene name='71/719201/Cv/3'>Synthetic gliadin peptide with HLA class II histocompatibility antigen α chain, β chain and ethylene glycol</scene> (PDB code [[1s9v]]). | ||
== Gluten Protein Complex == | == Gluten Protein Complex == | ||
| Line 29: | Line 31: | ||
| - | When the gluten peptide enters the HLA-DQ8 antigen binding cleft and makes contact, numerous | + | When the gluten peptide enters the HLA-DQ8 antigen binding cleft and makes contact, <scene name='71/719201/Hbonds_in_hla_dq8/1'> numerous weak interactions are made </scene>.<ref>Henderson, K. N., Tye-Din, J. A., Reid, H. H., Chen, Z., Borg, N. A., Beissbarth, T., . . . Anderson, R. P. (2007). A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity,27(1), 23-34. doi:10.1016/j.immuni.2007.05.015</ref> Notable contacts include: 16 direct hydrogen bonds, 24 water mediated hydrogen bonds, and four salt bridges. The side chains of two glutamic acids and one phenylalanine buried deep in pockets of the complex serve to anchor the peptide within the antigen-binding groove. A proline and serine can be found in shallow pockets with their side chain serving as minor anchor residues to the peptide. |
== Treatments == | == Treatments == | ||
| Line 35: | Line 37: | ||
[http://en.wikipedia.org/wiki/Prolyl_endopeptidase Prolyl endopeptidases] (PEPs) are a family of [http://en.wikipedia.org/wiki/Serine_protease Serine proteases] that help accelerate the breakdown of peptides by cleaving after proline residues. Since gluten is a proline-rich complex, these enzymes are being explored as a therapy to help treat individuals with celiac disease, since these individuals aren’t able to break down gluten naturally. <ref name="shan">Shan, L., I. I. Mathews, and C. Khosla. "Structural and Mechanistic Analysis of Two Prolyl nEndopeptidases: Role of Interdomain Dynamics in Catalysis and Specificity." Proceedings of the National Academy of Sciences 102.10 (2005): 3599-604. Web.</ref> | [http://en.wikipedia.org/wiki/Prolyl_endopeptidase Prolyl endopeptidases] (PEPs) are a family of [http://en.wikipedia.org/wiki/Serine_protease Serine proteases] that help accelerate the breakdown of peptides by cleaving after proline residues. Since gluten is a proline-rich complex, these enzymes are being explored as a therapy to help treat individuals with celiac disease, since these individuals aren’t able to break down gluten naturally. <ref name="shan">Shan, L., I. I. Mathews, and C. Khosla. "Structural and Mechanistic Analysis of Two Prolyl nEndopeptidases: Role of Interdomain Dynamics in Catalysis and Specificity." Proceedings of the National Academy of Sciences 102.10 (2005): 3599-604. Web.</ref> | ||
| - | < | + | <table align="left"><tr><td> |
| - | A study was done to explore the structural features of two bacterial PEPs with the purpose of evaluating their ability to cleave proline-rich residues. One was isolated from [https://en.wikipedia.org/wiki/Myxococcus_xanthus ''Myxococcus xanthus'']with a bound enzyme inhibitor and one was isolated from [https://en.wikipedia.org/wiki/Sphingomonas ''Sphingomonas capsulata''] in an unbound state. Both PEPs have two domains: a catalytic binding site that forms interactions with the substrate and the propeller domain that acts as a sort of stabilizing clamp structure. <ref name="shan" /> | + | [[Image:Proteopedia article image 2.jpg]] |
| - | < | + | </td></tr><tr><td> |
| + | Image Copyright 2005, National Academy of Sciences<ref name="pnas">"Permission is not required to use original figures or tables for noncommercial and educational use",[https://www.pnas.org/page/about/rights-permissions Rights & Permissions], Proceedings of the National Aademy of Sciences, USA.</ref>. | ||
| + | </td></tr></table> | ||
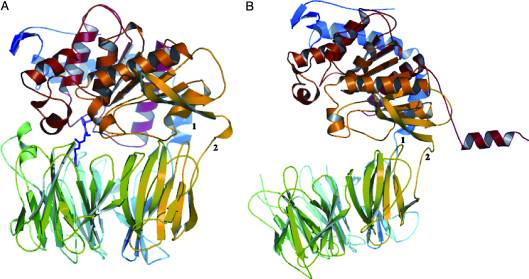
| + | A study was done to explore the structural features of two bacterial PEPs with the purpose of evaluating their ability to cleave proline-rich residues. One was isolated from [https://en.wikipedia.org/wiki/Myxococcus_xanthus ''Myxococcus xanthus'']with a <scene name='71/719201/Bound_pep/1'> bound enzyme inhibitor </scene> and one was isolated from [https://en.wikipedia.org/wiki/Sphingomonas ''Sphingomonas capsulata''] in an <scene name='71/719201/Unbound_pep/1'> unbound state </scene>. Both PEPs have two domains: a catalytic binding site that forms interactions with the substrate and the propeller domain that acts as a sort of stabilizing clamp structure. <ref name="shan" /> | ||
| + | |||
| + | <table align="left"><tr><td> | ||
| + | [[Image:Proteopedia article image 3.jpg]] | ||
| + | </td></tr><tr><td> | ||
| + | Image Copyright 2005, National Academy of Sciences<ref name="pnas">"Permission is not required to use original figures or tables for noncommercial and educational use",[https://www.pnas.org/page/about/rights-permissions Rights & Permissions], Proceedings of the National Aademy of Sciences, USA.</ref>. | ||
| + | </td></tr></table> | ||
| + | |||
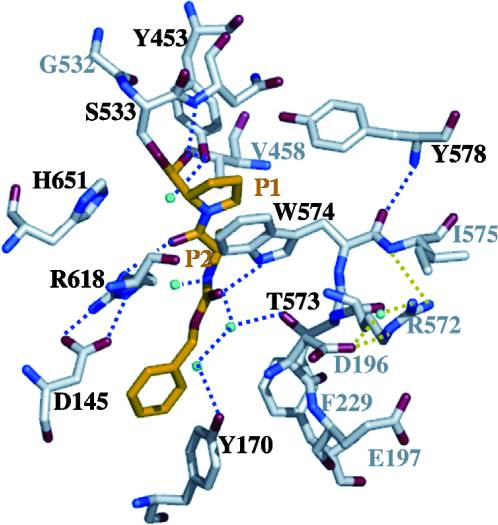
With further investigation of domain features of the PEP isolated from ''Myxococcus xanthus'', interactions were observed that give the enzyme its proline-cleaving properties. Interactions between the domains of the PEP isolated from ''Myxococcus xanthus'' and the inhibitor in the binding pocket, which is colored yellow. Residues from the catalytic domain are labeled in black with their hydrogen bonds represented by blue dashed lines and residues from the propeller domain are labeled in gray with the salt bridges represented by yellow dashed lines. <ref name="shan" /> | With further investigation of domain features of the PEP isolated from ''Myxococcus xanthus'', interactions were observed that give the enzyme its proline-cleaving properties. Interactions between the domains of the PEP isolated from ''Myxococcus xanthus'' and the inhibitor in the binding pocket, which is colored yellow. Residues from the catalytic domain are labeled in black with their hydrogen bonds represented by blue dashed lines and residues from the propeller domain are labeled in gray with the salt bridges represented by yellow dashed lines. <ref name="shan" /> | ||
| Line 48: | Line 60: | ||
</StructureSection> | </StructureSection> | ||
| + | == 3D structures of gliadin peptide== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | [[4gg6]] – wGLI alpha/beta peptide + antibody + T-cell receptor – wheat<br /> | ||
| + | [[4z7u]], [[4z7v]], [[4z7w]] – wGLI alpha-1 peptide + antibody + T-cell receptor <br /> | ||
| + | [[4ozf]], [[4ozg]], [[4ozh]], [[4ozi]] – wGLI alpha-2 peptide + antibody + T-cell receptor <br /> | ||
| + | [[1s9v]], [[2nna]] – GLI alpha-1 peptide + antibody - synthetic<br /> | ||
| + | [[4d8p]] – wGLI gamma peptide + antibody <br /> | ||
| + | |||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of gliadin peptide
Updated on 21-July-2025
4gg6 – wGLI alpha/beta peptide + antibody + T-cell receptor – wheat
4z7u, 4z7v, 4z7w – wGLI alpha-1 peptide + antibody + T-cell receptor
4ozf, 4ozg, 4ozh, 4ozi – wGLI alpha-2 peptide + antibody + T-cell receptor
1s9v, 2nna – GLI alpha-1 peptide + antibody - synthetic
4d8p – wGLI gamma peptide + antibody
References
- ↑ Celiac Disease: MedlinePlus. Retrieved October 27, 2015, from https://www.nlm.nih.gov/medlineplus/celiacdisease.html
- ↑ Celiac Disease. Retrieved October 27, 2015, from http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/celiac-disease/Pages/facts.aspx
- ↑ Go to Science. Retrieved October 27, 2015, from http://www.sciencemag.org/content/297/5590/2275.full
- ↑ 4.0 4.1 Maiuri, L., Ciacci, C., Ricciardelli, I., Vacca, L., Raia, V., Auricchio, S., . . . Londei, M. (2003). Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet, 362(9377), 30-37. doi:10.1016/S0140-6736(03)13803-2
- ↑ Mellins, E., & Stern, L. (n.d.). HLA-DM and HLA-DO, key regulators of MHC-ll processing and presentation. Current Opinion in Immunology, 26, 115-122. February 2014. http://www.sciencedirect.com/science/article/pii/S095279151300215X
- ↑ Kim, C., Quartsen, H., Bergsen, E., Khosla, C., & Sollid, L.. (n.d.). Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Cross Mark, 101(12), 4175-4179. March 2004 http://www.pnas.org/content/101/12/4175.figures-only
- ↑ Henderson, K. N., Tye-Din, J. A., Reid, H. H., Chen, Z., Borg, N. A., Beissbarth, T., . . . Anderson, R. P. (2007). A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity,27(1), 23-34. doi:10.1016/j.immuni.2007.05.015
- ↑ 8.0 8.1 8.2 8.3 8.4 Shan, L., I. I. Mathews, and C. Khosla. "Structural and Mechanistic Analysis of Two Prolyl nEndopeptidases: Role of Interdomain Dynamics in Catalysis and Specificity." Proceedings of the National Academy of Sciences 102.10 (2005): 3599-604. Web.
- ↑ 9.0 9.1 "Permission is not required to use original figures or tables for noncommercial and educational use",Rights & Permissions, Proceedings of the National Aademy of Sciences, USA.
- ↑ Matysiak-Budnik, T., Candalh, C., Cellier, C., Dugave, C., Namane, A., Vidal-Martinez, T., . . . Heyman, M. (2005). Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology,129(3), 786-796. doi:10.1053/j.gastro.2005.06.016
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Premal Patel, Eric Martz, Alexander Berchansky, Devin Joseph, Ben Horansky, Gunnar Reiske, Katlin Cannon, Joel L. Sussman, Jaime Prilusky