Sandbox Reserved 1358
From Proteopedia
| (16 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_HLSC322}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_HLSC322}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | == | + | ==GLUT3 / SLC2A3== |
| - | <StructureSection load=' | + | <StructureSection load='5c65' size='340' side='right' caption='Structure of DNA' scene=''> |
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| + | The GLUT3 protein, encoded by the SLC2A3 gene in humans, found in mammalian cells, allows for the transport of the glucose molecule across the plasma membrane. It is especially prevalent in the axons of nerve cells in brain tissue. In neurons themselves, GLUT3 is the main glucose transporter that is utilized. In the human body, of the first four glucose transporters discovered (GLUT1, GLUT2, GLUT3, and GLUT4), GLUT3 has a higher glucose affinity and transport capacity, which facilitates its use in glucose transport in neurons. This is significant because the glucose levels surrounding the neuron are five times lower than those found in serum (blood). GLUT3 is also present in sperm, embryos, white blood cells and carcinoma cell lines. | ||
| + | |||
| + | == Structure == | ||
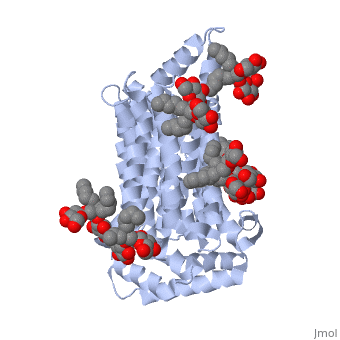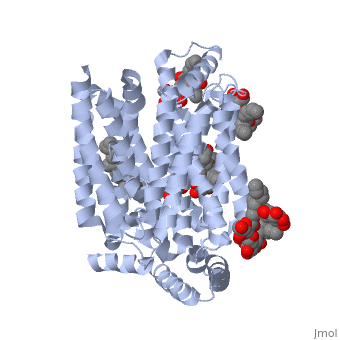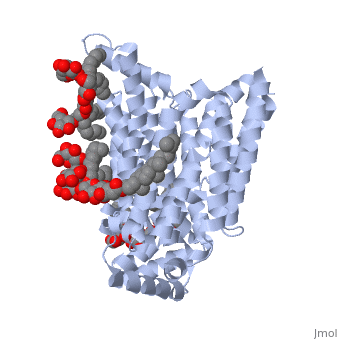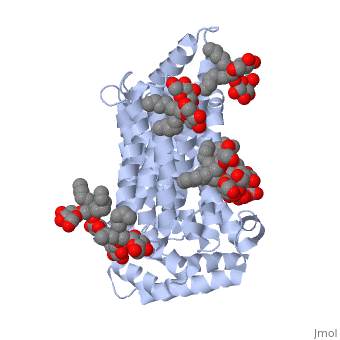
| + | GLUT3 transport protein is composed of 481 amino acids, <scene name='77/777678/Two_protein_chains/1'>two alpha helical protein chains</scene>, and two different ligands, ligand <scene name='77/777678/Ligand_y01/1'>Y01</scene> and ligand <scene name='77/777678/Ligand_37x/1'>37X</scene>. On the protein, one ligand Y01, known as Cholesterol hemisuccinate, is attached to chain a, and one to chain b. Ligand 37X is called Octyl Glucose Neopentyl Glycol, and there are 6 of these ligands on chain a and 3 on chain b. | ||
== Disease == | == Disease == | ||
| + | Correlations between low levels of glucose transport proteins and abnormal hyperphosphorylation of tau in Alzheimer Disease. Individuals with Alzheimer Disease have decreased levels of GLUT3 and GLUT1 in their brain. | ||
| - | == | + | </StructureSection> |
| + | == References == | ||
| + | Kayano, T., Fukumoto, H., Eddy, R. L., Fan, Y. S., Byers, M. G., Shows, T. B., & Bell, G. I. (n.d.). Cell Glucose Transport and Glucose Handling During Fetal and Neonatal Development. Retrieved from Journal of Biological Chemistry website: http://www.jbc.org/content/263/30/15245.short | ||
| - | + | Liu, Y., Liu, F., Iqbal, K., Grundke-Iqbal, I., & Gong, C.-X. (2008). Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Letters, 582(2), 359–364. http://doi.org/10.1016/j.febslet.2007.12.035 | |
| - | + | Simmons, R. A. (2017). Cell Glucose Transport and Glucose Handling During Fetal and Neonatal Development. Retrieved from Science Direct website: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/glut3 | |
| - | + | Simpson, I. A., Dwyer, D., Malide, D., Moley, K. H., Travis, A., & Vannucci, S. J. (2008). The facilitative glucose transporter GLUT3: 20 years of distinction. American Journal of Physiology - Endocrinology and Metabolism, 295(2), E242–E253. http://doi.org/10.1152/ajpendo.90388.2008 | |
| - | + | ||
| - | + | ||
Current revision
| This Sandbox is Reserved from January through July 31, 2018 for use in the course HLSC322: Principles of Genetics and Genomics taught by Genevieve Houston-Ludlam at the University of Maryland, College Park, USA. This reservation includes Sandbox Reserved 1311 through Sandbox Reserved 1430. |
To get started:
More help: Help:Editing |
GLUT3 / SLC2A3
| |||||||||||
References
Kayano, T., Fukumoto, H., Eddy, R. L., Fan, Y. S., Byers, M. G., Shows, T. B., & Bell, G. I. (n.d.). Cell Glucose Transport and Glucose Handling During Fetal and Neonatal Development. Retrieved from Journal of Biological Chemistry website: http://www.jbc.org/content/263/30/15245.short
Liu, Y., Liu, F., Iqbal, K., Grundke-Iqbal, I., & Gong, C.-X. (2008). Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Letters, 582(2), 359–364. http://doi.org/10.1016/j.febslet.2007.12.035
Simmons, R. A. (2017). Cell Glucose Transport and Glucose Handling During Fetal and Neonatal Development. Retrieved from Science Direct website: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/glut3
Simpson, I. A., Dwyer, D., Malide, D., Moley, K. H., Travis, A., & Vannucci, S. J. (2008). The facilitative glucose transporter GLUT3: 20 years of distinction. American Journal of Physiology - Endocrinology and Metabolism, 295(2), E242–E253. http://doi.org/10.1152/ajpendo.90388.2008