We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
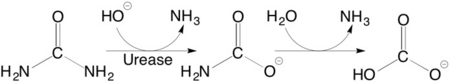
Urease
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 86: | Line 86: | ||
{{Clear}} | {{Clear}} | ||
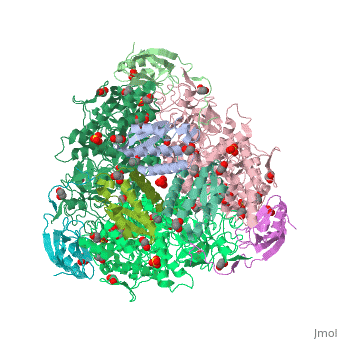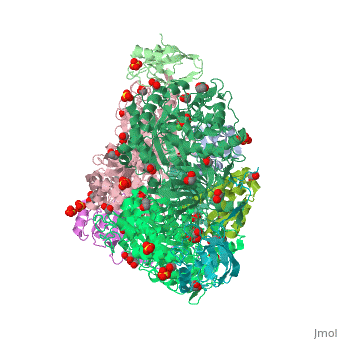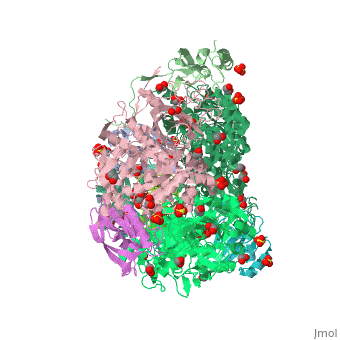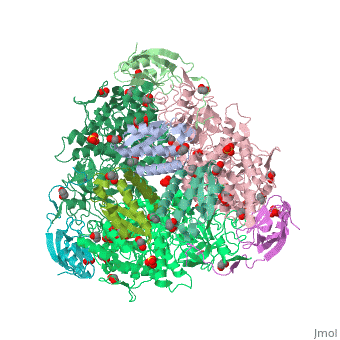
The present study consistently supports an interaction of fluoride with the nickel centres in the urease active site in which <scene name='59/596313/Cv/17'>one fluoride competitively binds</scene> (<span style="color:salmon;background-color:black;font-weight:bold;">colored in salmon</span>) to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while <scene name='59/596313/Cv/18'>another fluoride uncompetitively substitutes</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">colored in cyan</span>) the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea. | The present study consistently supports an interaction of fluoride with the nickel centres in the urease active site in which <scene name='59/596313/Cv/17'>one fluoride competitively binds</scene> (<span style="color:salmon;background-color:black;font-weight:bold;">colored in salmon</span>) to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while <scene name='59/596313/Cv/18'>another fluoride uncompetitively substitutes</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">colored in cyan</span>) the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea. | ||
| + | =3D structures of urease= | ||
| + | [[Urease 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
__NOTOC__ | __NOTOC__ | ||
| - | =3D structures of urease= | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *Urease | ||
| - | |||
| - | **[[2kau]], [[1kra]], [[1fwj]], [[1ejx]], [[1ejw]], [[4ep8]] – KaUA α+β+γ chains – ''Klebsiella aerogenes''<br /> | ||
| - | **[[1ef2]] - KaUA α+β+γ chains Mn substituted<br /> | ||
| - | **[[1krb]], [[1krc]], [[1fwa]], [[1fwb]], [[1fwc]], [[1fwd]], [[1fwf ]], [[1fwg]], [[1fwh]], [[1fwi]] – KaUA α (mutant) +β (mutant) +γ (mutant) chains<br /> | ||
| - | **[[1a5k]], [[1a5l]], [[1a5m]], [[1ejr]], [[1ejs]], [[1ejt]], [[1eju]], [[1ejv]] - KaUA α+β+γ (mutant) chains<br /> | ||
| - | **[[2ubp]] - BpUA α+β+γ chains – ''Bacillus pasteurii''<br /> | ||
| - | **[[1e9z]] - HpUA α+β chains – ''Helicobacter pylori''<br /> | ||
| - | **[[3qga]], [[3qgk]] - UA β/γ chains Fe containing – ''Helicobacter mustelae''<br /> | ||
| - | **[[2fvh]] - UA γ chain – ''Mycobacterium tuberculosis''<br /> | ||
| - | **[[3la4]] – hbUA – horse bean<br /> | ||
| - | **[[2mm8]] – hbUA – NMR<br /> | ||
| - | **[[4epb]], [[4epd]], [[4epe]] - UA α+β+γ chains – ''Enterobacter aerogenes''<br /> | ||
| - | **[[4ac7]], [[4ceu]] - SpUA α+β+γ chains – ''Sporosarcina pasteurii''<br /> | ||
| - | **[[4fur]] - UA γ2 chain – ''Enterobacter melitensis''<br /> | ||
| - | **[[4g7e]] - UA – pigeon pea<br /> | ||
| - | **[[4gy7]] - jbUA – jack bean<br /> | ||
| - | |||
| - | *Urease binary complex | ||
| - | **[[1a5n]], [[1a5o]] - KaUA α+β+γ (mutant) chains + formate<br /> | ||
| - | **[[1fwe]] – KaUA α (mutant) +β (mutant) +γ (mutant) chains + acetohydroxamic acid<br /> | ||
| - | **[[1ubp]] - BpUA α+β+γ chains + mercaptoethanol <br /> | ||
| - | **[[3ubp]] - BpUA α+β+γ chains + diamidophosphate<br /> | ||
| - | **[[4ubp]] - BpUA α+β+γ chains + acetohydroxamic acid<br /> | ||
| - | **[[1ie7]] - BpUA α+β+γ chains + phosphate<br /> | ||
| - | **[[1s3t]] - BpUA α+β+γ chains + borate<br /> | ||
| - | **[[4z42]] - UA α+β+γ chains + Ni – ''Yersinia enterocolitica''<br /> | ||
| - | **[[1e9y]] - HpUA α+β chains + acetohydroxamic acid<br /> | ||
| - | **[[4cex]] - SpbUA α+β+γ chains + F <br /> | ||
| - | **[[5fsd]], [[5fse]], [[5a6t]], [[5g4h]], [[5ol4]] - SpUA α+β+γ chains + Ni + inhibitor<br /> | ||
| - | **[[4goa]] - jbUA + F <br /> | ||
| - | **[[4h9m]] - jbUA + acetohydroxamic acid<br /> | ||
| - | }} | ||
=Additional Resources= | =Additional Resources= | ||
For additional information on Urinary Tract Infection, See: [[1tr7]] <br /> | For additional information on Urinary Tract Infection, See: [[1tr7]] <br /> | ||
Current revision
| |||||||||||
Additional Resources
For additional information on Urinary Tract Infection, See: 1tr7
For additional information on Helicobacter Pylori, See: 1e9z
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 PMID: PMC2443974
- ↑ http://www.jbc.org/content/277/35/e23.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&searchid=1130442887043_7599&stored_search=&FIRSTINDEX=60&tocsectionid=Classics&sortspec=PUBDATE_SORTDATE+desc
- ↑ Andrews, R. K., Blakeley, R. L. & Zerner, B. (1984). Urea and urease. Adv. Inorg. Biochem. 6, 245–283.
- ↑ Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480.
- ↑ Moncrief, M. C. & Hausinger, R. P. (1996). Nickel incorporation into urease. In Mechanisms of Metallo- center Assembly (Hausinger, R. P., Eichhorn, G. L. & Marzilli, L. G., eds), pp. 151–171, Elsevier Press, New York, NY.
- ↑ 6.0 6.1 Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.
- ↑ Polacco, J. C. & Holland, M. A. (1993). Roles of urease in plant cells. Int. Rev. Cytol. 145, 65–103.
- ↑ 8.0 8.1 http://en.wikipedia.org/wiki/Urease
- ↑ 9.0 9.1 9.2 Mobley, H. L. T., Island, M. D. & Hausinger, R. P. (1995). Molecular biology of microbial ureases. Microbiol. Rev. 59, 451–480.
- ↑ http://www.cell.com/structure/abstract/S0969-2126(99)80026-4#.
- ↑ Cicmanec JF, Helmers SL, Evans AT. Office practice survey of urease positive bacterial pathogens causing urinary tract infections. Urology. 1980 Sep;16(3):274-6. PMID:6999699
- ↑ Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480.
- ↑ Becker-Ritt, A. B., Martinelli, A. H. S., Mitidieri, S., Feder, V., Wassermann, G. E., Santi, L. et al. (2007). Antifungal activity of plant and bacterial ureases. Toxicon, 50, 971–983.
- ↑ 14.0 14.1 Follmer, C., Real-Guerra, R., Wassermann, G. E., Olivera-Severo, D. & Carlini, C. R. (2004). Jackbean, soybean and Bacillus pasteurii ureases—biological effects unrelated to ureolytic activity. Eur. J. Biochem. 271, 1357–1363.
- ↑ Karplus, P. A., Pearson, M. A. & Hausinger, R. P. (1997). 70 years of crystalline urease: what have we learnt? Acc. Chem. Res. 30, 330–337.
- ↑ Benini, S., Rypneiwski, W. R., Wilson, K. S., Meletti, S., Ciurli, S. & Mangani, S. (1999). A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure, 7, 205–216.
- ↑ 17.0 17.1 http://tonga.usip.edu/jsnow/chem348/recitation8.pdf
- ↑ http://emedicine.medscape.com/article/1174503-overview
- ↑ http://www.nucdf.org/ucd_treatment.htm
- ↑ Benini S, Kosikowska P, Cianci M, Mazzei L, Vara AG, Berlicki L, Ciurli S. The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design. J Biol Inorg Chem. 2013 Mar;18(3):391-9. doi: 10.1007/s00775-013-0983-7. Epub, 2013 Feb 15. PMID:23412551 doi:10.1007/s00775-013-0983-7
- ↑ Kcx - Lysine NZ-carboxylic acid
- ↑ Zambelli B, Banaszak K, Merloni A, Kiliszek A, Rypniewski W, Ciurli S. Selectivity of Ni(II) and Zn(II) binding to Sporosarcina pasteurii UreE, a metallochaperone in the urease assembly: a calorimetric and crystallographic study. J Biol Inorg Chem. 2013 Dec;18(8):1005-17. doi: 10.1007/s00775-013-1049-6. Epub, 2013 Oct 15. PMID:24126709 doi:http://dx.doi.org/10.1007/s00775-013-1049-6
- ↑ Ligabue-Braun R, Real-Guerra R, Carlini CR, Verli H. Evidence-based docking of the urease activation complex. J Biomol Struct Dyn. 2012 Sep 10. PMID:22962938 doi:10.1080/07391102.2012.713782
- ↑ Benini S, Cianci M, Mazzei L, Ciurli S. Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics. J Biol Inorg Chem. 2014 Aug 12. PMID:25113581 doi:http://dx.doi.org/10.1007/s00775-014-1182-x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Andrea Graydon, Alexander Berchansky, David Canner, OCA