Sandbox Reserved 1508
From Proteopedia
| (One intermediate revision not shown.) | |||
| Line 9: | Line 9: | ||
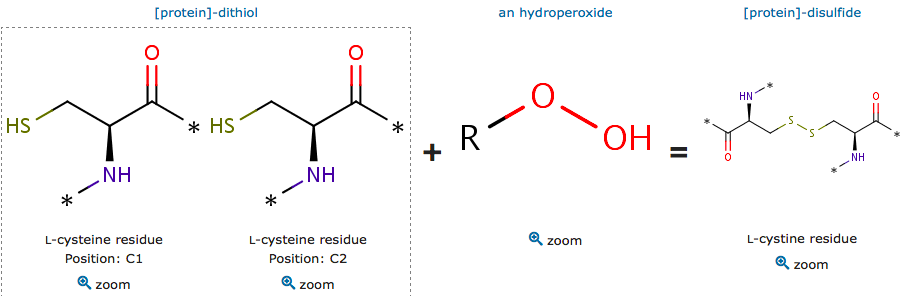
Peroxiredoxins are peroxidases which catalyze the reduction of peroxides (organic peroxide H2O2 or organic hydroperoxides). | Peroxiredoxins are peroxidases which catalyze the reduction of peroxides (organic peroxide H2O2 or organic hydroperoxides). | ||
| - | |||
== Biological function == | == Biological function == | ||
| Line 28: | Line 27: | ||
== Catalytic site == | == Catalytic site == | ||
| - | The article architecture in peroxiredoxins: a case study on ''Mycobacterium tuberculosis'' AhpE (Zeida et al, 2015) describe | + | The article architecture in peroxiredoxins: a case study on ''Mycobacterium tuberculosis'' AhpE (Zeida et al, 2015) describe <scene name='80/802682/Cys45/1'>the active site</scene> of the protein 5C04 by using the model ''Mycobacterium tuberculosis'' AhpE (alkyl hydroperoxide reductases E). Researchers noticed that cysteine is not essential for the binding of H2O2 to the active site of peroxiredoxin. There are conserved residues in the active site which are important for H2O2 binding and reduction: the threonine from the PxxxTxxC sequence motif and an arginine distant in a sequence but close to the active site (Zeida et al, 2015). The conserved arginine plays a pivotal role in bringing the oxygen of the peroxide closer to the catalytic site, weakening the O–O bond and stabilizing the transition state between the proximal O (Zeida et al, 2015). |
At the active site of the enzyme, a pyrodoxal-phosphate cofactor is covalently linked to the Lysine 51 an invariant residue. A parallel β-sheet associated with three α-helices are part of the N terminal domain (residues 46 to 153). Two of those α-helices are part of the dimer interface and the third one is partly forming the entrance of the active site as on the other side of the β-sheet. On the other hand the C-terminal domain is made of 6-stranded mixed β-sheets surrounded by four α-helices (two on each sides) and of residues with a unique insertion of eight amino acids within them. (Ågren et al, 2008) | At the active site of the enzyme, a pyrodoxal-phosphate cofactor is covalently linked to the Lysine 51 an invariant residue. A parallel β-sheet associated with three α-helices are part of the N terminal domain (residues 46 to 153). Two of those α-helices are part of the dimer interface and the third one is partly forming the entrance of the active site as on the other side of the β-sheet. On the other hand the C-terminal domain is made of 6-stranded mixed β-sheets surrounded by four α-helices (two on each sides) and of residues with a unique insertion of eight amino acids within them. (Ågren et al, 2008) | ||
| Line 50: | Line 49: | ||
According to researches on biosynthetic pathway in ''Mycobacterium tuberculosis'' (Burns et al, 2008), there are three pathways implicated cysteine in this disease: the sulfide dependent pathway, the cystathionine pathway and the CysO-thiocarboxylate pathway. For the CysO depending pathway, transcriptional profile analysis shown that cysM and cysO are upregulated under oxidative stress conditions. Moreover, the thiocarboxylate are much more resistant to oxidation than thiols. Thus, when the disease occurs, the environment becomes highly oxidizing due to the macrophages, leads to the cysteine biosynthesis. The CysO-thiocarboxylate evolves as an oxidation resistant form of sulfide, thiol is favored for the cysteine biosynthesis. | According to researches on biosynthetic pathway in ''Mycobacterium tuberculosis'' (Burns et al, 2008), there are three pathways implicated cysteine in this disease: the sulfide dependent pathway, the cystathionine pathway and the CysO-thiocarboxylate pathway. For the CysO depending pathway, transcriptional profile analysis shown that cysM and cysO are upregulated under oxidative stress conditions. Moreover, the thiocarboxylate are much more resistant to oxidation than thiols. Thus, when the disease occurs, the environment becomes highly oxidizing due to the macrophages, leads to the cysteine biosynthesis. The CysO-thiocarboxylate evolves as an oxidation resistant form of sulfide, thiol is favored for the cysteine biosynthesis. | ||
| - | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
The protein 5C04
| |||||||||||
References
Ågren, Daniel, Robert Schnell, Wulf Oehlmann, Mahavir Singh, et Gunter Schneider. « Cysteine Synthase (CysM) of Mycobacterium Tuberculosis Is an O -Phosphoserine Sulfhydrylase: EVIDENCE FOR AN ALTERNATIVE CYSTEINE BIOSYNTHESIS PATHWAY IN MYCOBACTERIA ». Journal of Biological Chemistry 283, nᵒ 46 (14 novembre 2008): 31567‑74. https://doi.org/10.1074/jbc.M804877200.
Burns, Kristin E., Sabine Baumgart, Pieter C. Dorrestein, Huili Zhai, Fred W. McLafferty, et Tadhg P. Begley. « Reconstitution of a New Cysteine Biosynthetic Pathway in Mycobacterium t Uberculosis ». Journal of the American Chemical Society 127, nᵒ 33 (août 2005): 11602‑3. https://doi.org/10.1021/ja053476x.
Pedre, Brandán, Laura A. H. van Bergen, Anna Palló, Leonardo A. Rosado, Veronica Tamu Dufe, Inge Van Molle, Khadija Wahni, et al. « The Active Site Architecture in Peroxiredoxins: A Case Study on Mycobacterium Tuberculosis AhpE ». Chemical Communications 52, nᵒ 67 (2016): 10293‑96. https://doi.org/10.1039/C6CC02645A.
Rhee, Sue Goo, et Hyun Ae Woo. « Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H 2 O 2 , and Protein Chaperones ». Antioxidants & Redox Signaling 15, nᵒ 3 (août 2011): 781‑94. https://doi.org/10.1089/ars.2010.3393.
Zeida, Ari, Aníbal M. Reyes, Pablo Lichtig, Martín Hugo, Diego S. Vazquez, Javier Santos, F. Luis González Flecha, Rafael Radi, Dario A. Estrin, et Madia Trujillo. « Molecular Basis of Hydroperoxide Specificity in Peroxiredoxins: The Case of AhpE from Mycobacterium Tuberculosis ». Biochemistry 54, nᵒ 49 (15 décembre 2015): 7237‑47. https://doi.org/10.1021/acs.biochem.5b00758.