Sandbox Reserved 1482
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== '''Coagulation Factor VIII (3cdz)''' == | == '''Coagulation Factor VIII (3cdz)''' == | ||
<StructureSection load='3cdz' size='340' side='right' caption='The human coagulation factor VIII' scene=''> | <StructureSection load='3cdz' size='340' side='right' caption='The human coagulation factor VIII' scene=''> | ||
| - | '''The human Factor VIII''', also known as anti-hemophilic factor (AHF), is an essential blood-clotting protein <ref name="wikipedia">https://en.wikipedia.org/wiki/Factor_VIII [11.01.2019]</ref>. It consists of 2332 residues <ref name="Ngo">PMID: 18400180</ref> | + | '''The human Factor VIII''', also known as anti-hemophilic factor (AHF), is an essential blood-clotting protein <ref name="wikipedia">https://en.wikipedia.org/wiki/Factor_VIII [11.01.2019]</ref>. It consists of 2332 residues <ref name="Ngo">PMID: 18400180</ref>. Its gene is located on the X chromosome <ref name="wikipedia" /><ref name="Antonarakis">PMID: 8578479</ref>. |
Factor VIII is produced inside the liver (by the sinusoidal cells) and outside (by the endothelial cells) and acts in the intrinsic pathway of blood coagulation <ref name="wikipedia" />. It is actually the lack or the deficiency of the factor VIII (which is a plasma glycoprotein) that causes a bleeding disorder: hemophilia A <ref name="Ngo" />. | Factor VIII is produced inside the liver (by the sinusoidal cells) and outside (by the endothelial cells) and acts in the intrinsic pathway of blood coagulation <ref name="wikipedia" />. It is actually the lack or the deficiency of the factor VIII (which is a plasma glycoprotein) that causes a bleeding disorder: hemophilia A <ref name="Ngo" />. | ||
| Line 40: | Line 40: | ||
====Secondary Structure==== | ====Secondary Structure==== | ||
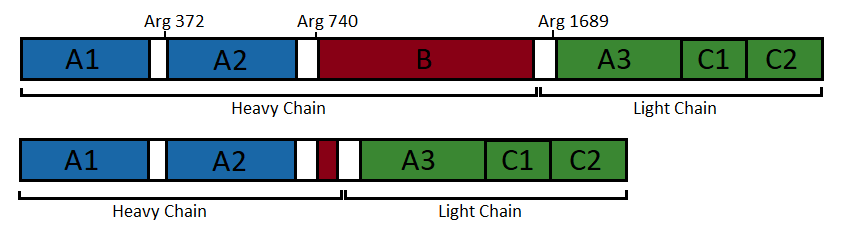
Factor VIII protein is composed of six globular domains: A<sub>1</sub>-A<sub>2</sub>-B-A<sub>3</sub>-C<sub>1</sub>-C<sub>2</sub> and contains one Ca<sup>2+</sup> and two Cu<sup>2+</sup> ions. It has a molecular weight of 330 kDa <ref name="Ngo" /><ref name="El" /><ref name="uni" />. | Factor VIII protein is composed of six globular domains: A<sub>1</sub>-A<sub>2</sub>-B-A<sub>3</sub>-C<sub>1</sub>-C<sub>2</sub> and contains one Ca<sup>2+</sup> and two Cu<sup>2+</sup> ions. It has a molecular weight of 330 kDa <ref name="Ngo" /><ref name="El" /><ref name="uni" />. | ||
| + | |||
| + | In the following, the structure of an engineered protein is further described. This protein has no B domain to mimic the active factor VIIIa. In addition, it is more amenable to structural studies because it shows higher stability expression levels and structural homogenity <ref name="Ngo" /><ref name="toole">Toole JJ, Pittman DD, Orr EC, Murtha P, Wasley LC & Kaufman RJ. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for ''in vitro'' procoagulant activity. Proceedings of the National Academy of Sciences. 1986 Aug; 83(16): 5939-5942. PMID: 3016730 doi https://doi.org/10.1073/pnas.83.16.5939</ref>. | ||
The three A domains are homologous to the A domains of the copper-binding protein [[Ceruloplasmin]] <ref name="wikipedia" /><ref name="El" />. Together, they form a triangular heterotrimer where the A<sub>1</sub> and A<sub>3</sub> domains interact with the C<sub>2</sub> and C<sub>1</sub> domains, respectively <ref name="Ngo" />. | The three A domains are homologous to the A domains of the copper-binding protein [[Ceruloplasmin]] <ref name="wikipedia" /><ref name="El" />. Together, they form a triangular heterotrimer where the A<sub>1</sub> and A<sub>3</sub> domains interact with the C<sub>2</sub> and C<sub>1</sub> domains, respectively <ref name="Ngo" />. | ||
| Line 45: | Line 47: | ||
The C domains belong to the phospholipid-binding discoidin domain family <ref name="wikipedia" />. They are adjacent at the base of the triangular heterotrimer. Moreover, C<sub>1</sub> and C<sub>2</sub> domains are structurally homologous and they have the ability to bind the membrane. Indeed, both C domain protrude three β-hairpin loops with hydrophobic and basic residues in the same direction. Thanks to these loops the factor VIII might interact with the phospholipid bilayer. <ref name="Ngo" /> | The C domains belong to the phospholipid-binding discoidin domain family <ref name="wikipedia" />. They are adjacent at the base of the triangular heterotrimer. Moreover, C<sub>1</sub> and C<sub>2</sub> domains are structurally homologous and they have the ability to bind the membrane. Indeed, both C domain protrude three β-hairpin loops with hydrophobic and basic residues in the same direction. Thanks to these loops the factor VIII might interact with the phospholipid bilayer. <ref name="Ngo" /> | ||
| - | Factor VIIIa is obtained by cleavage and release of the B domain <ref name="wikipedia" /><ref name="Ngo" /><ref name="toole" | + | Factor VIIIa is obtained by cleavage and release of the B domain <ref name="wikipedia" /><ref name="Ngo" /><ref name="toole" />. Although factor VIIIa can be formed from at least two cleavages involving Arg372 and Arg1689, fully active factor VIIIa is obtained only after a third cleavage at Arg740 <ref name="Ngo" />. |
| Line 53: | Line 55: | ||
• The <scene name='80/802656/Light_chain/2'>light chain</scene> has a molecular weight of 80 kDa and is composed of 684 amino acids <ref name="Binhoreau" />. It contains two domains: a unique A domain of 371 amino acids and a duplicated C domain of 153 amino acids and 160 amino acids, respectively <ref name="Binhoreau" />. These domains are ranked in the following order A<sub>3</sub>-C<sub>1</sub>-C<sub>2</sub> <ref name="wikipedia" /><ref name="Binhoreau" />. It is composed of 42 % irregular structure, 36 % β-strands, and 22 % α-helices <ref name="Binhoreau" />. The C<sub>1</sub> and C<sub>2</sub> domains are defined by a distorted β barrel, while A<sub>3</sub>, as well as A<sub>1</sub> and A<sub>2</sub>, is composed of two connected β barrels <ref name="Ngo" />. This chain also contains of the major binding site of von Willebrand Factor at its N-terminus <ref name="Binhoreau" />. | • The <scene name='80/802656/Light_chain/2'>light chain</scene> has a molecular weight of 80 kDa and is composed of 684 amino acids <ref name="Binhoreau" />. It contains two domains: a unique A domain of 371 amino acids and a duplicated C domain of 153 amino acids and 160 amino acids, respectively <ref name="Binhoreau" />. These domains are ranked in the following order A<sub>3</sub>-C<sub>1</sub>-C<sub>2</sub> <ref name="wikipedia" /><ref name="Binhoreau" />. It is composed of 42 % irregular structure, 36 % β-strands, and 22 % α-helices <ref name="Binhoreau" />. The C<sub>1</sub> and C<sub>2</sub> domains are defined by a distorted β barrel, while A<sub>3</sub>, as well as A<sub>1</sub> and A<sub>2</sub>, is composed of two connected β barrels <ref name="Ngo" />. This chain also contains of the major binding site of von Willebrand Factor at its N-terminus <ref name="Binhoreau" />. | ||
| + | |||
| + | |||
| + | Figure 1: Domain organisation of the uncleaved coagulation factor VIII (top) and the engineered factor without B domain (bottom). (Figure adapted from <ref name="Ngo" />) | ||
| + | |||
| + | [[Image:Domain organisation factorVIII (un)cleaved.png]] | ||
Both chains are non-covalently associated through to a calcium ion to form the active heterodimer <ref name="Ngo" /><ref name="Binhoreau" />. This complex is the pro-coagulant factor VIIIa <ref name="wikipedia" />. | Both chains are non-covalently associated through to a calcium ion to form the active heterodimer <ref name="Ngo" /><ref name="Binhoreau" />. This complex is the pro-coagulant factor VIIIa <ref name="wikipedia" />. | ||
| Line 58: | Line 65: | ||
====Ligands==== | ====Ligands==== | ||
| - | + | The calcium ion (Ca<sup>2+</sup>) and the copper ion (Cu<sup>2+</sup>) are both ligands the factor VIII is able to bind to <ref name="pdb" />. More precisely, in factor VIII there are two copper ions and their binding sites are located internally within the <scene name='80/802656/A3cu/1'>A3</scene> and the <scene name='80/802656/A1/1'>A1</scene> domain. The <scene name='80/802656/A1/1'>A1</scene> domain binds another ligand, a <scene name='80/802656/A1ca/1'>calcium ion</scene>, bound to its own binding site. <ref name="Ngo" /> | |
| - | + | ||
| + | One other molecule can be found on this protein: N-acetyl-D-glucosamine. This molecule is covalently bound to Asn residues of the protein during the maturation process in the endoplasmic reticulum and the Golgi apparatus <ref name="Lenting">Lenting PJ, Pegon JN, Christophe OD, Denis CV. Factor VIII and von Willebrand factor – too sweet for their own good. Haemophilia. 2010 June; 16(Suppl. 5), 194–199. PMID: 20590881 doi: https://doi.org/10.1111/j.1365-2516.2010.02320.x</ref>. N-acetyl-D-glucosamine is not a ligand since it is not a specific substrate that binds a specific site in the protein. Indeed many proteins have such a glycolysation on their asparagine residues <ref name="Apweiler">Apweiler R, Hermjakob H,Sharon N.On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database.Biochimica et Biophysica Acta (BBA)-General Subjects.1999 Dec; 1473(1), 4-8.PMID: 10580125 doi: 10.1016/s0304-4165(99)00165-8</ref>. N-acetyl-D-glucosamine is a post-translational modifications and may be different depending on the physiological context <ref name="Helenius">Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001 Mar, 291(5512), 2364-2369 doi:10.1126/science.291.5512.2364 </ref><ref>https://pubchem.ncbi.nlm.nih.gov/compound/439174 [25.06.2019]</ref>. | ||
| + | The alpha-D-mannose molecule, present in the structure shown here, might also be a posttranslational modification, since it is a sugar that can be establish N-type bonds<ref>https://en.wikipedia.org/wiki/Mannose [25.06.2019]</ref>. Factor VIII is thus a glycoprotein <ref name="Apweiler" />. | ||
| + | |||
| Line 76: | Line 86: | ||
====Inheritance==== | ====Inheritance==== | ||
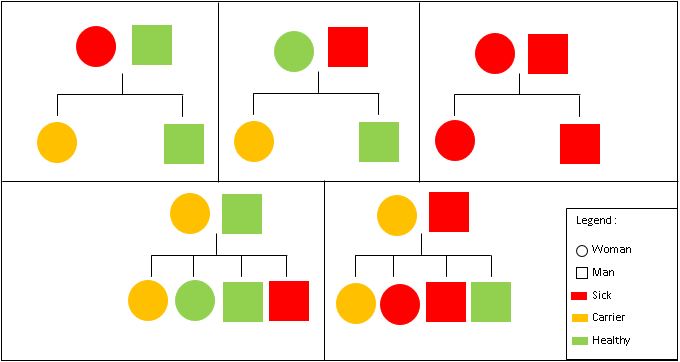
| - | Hemophilia A is inherited in an X-linked recessive manner. This means that if a son inherits an X chromosome carrying hemophilia from his mother, he will have hemophilia. By contrast, daughters, even if they inherit one hemophilia allele, | + | Hemophilia A is inherited in an X-linked recessive manner. This means that if a son inherits an X chromosome carrying hemophilia from his mother, he will have hemophilia. By contrast, daughters, even if they inherit one hemophilia allele, can compensate it with their second healthy X chromosome. As a result, women only rarely have symptoms, but women that are carriers, may pass the gene on to their children (50% chance per pregnancy) <ref name="Konkle" />. |
| - | + | Figure 2: Hemophilia Inheritance | |
[[Image:Hemophilia_inheritance.JPG|Hemophilia Inheritance]] | [[Image:Hemophilia_inheritance.JPG|Hemophilia Inheritance]] | ||
Current revision
Coagulation Factor VIII (3cdz)
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 https://en.wikipedia.org/wiki/Factor_VIII [11.01.2019]
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 Ngo JC, Huang M, Roth DA, Furie BC, Furie B. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008 Apr;16(4):597-606. PMID:18400180 doi:10.1016/j.str.2008.03.001
- ↑ 3.0 3.1 Antonarakis SE. Molecular genetics of coagulation factor VIII gene and hemophilia A. Thromb Haemost. 1995 Jul;74(1):322-8. PMID:8578479
- ↑ Ragni MV. Mimicking Factor VIII to Manage the Factor VIII–Deficient State. The New England journal of medicine. 2018 Aug; 379(9): 880-882. doi: 10.1056/NEJMe1808789
- ↑ Patek AJ & Taylor FHL. Hemophilia. II. Some properties of a substance obtained from normal human plasma effective in accelerating the coagulation of hemophilic blood. The Journal of clinical investigation. 1937 Jan; 16(1): 113-124. PMID: 16694450 doi: 10.1172/JCI100829
- ↑ Dallman PR & Pool JG. Treatment of hemophilia with factor VIII concentrates. New England Journal of Medicine. 1968 Jan ; 278(4): 199-202. PMID: 5711341 doi: 10.1056/NEJM196801252780406
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 El Khorassani M & Benkirane AN. Le facteur VIII coagulant. Médecine du Maghreb. 1996; 55: 11-13.
- ↑ Ljung RC. Prevention and management of bleeding episodes in children with hemophilia. Pediatric Drugs. 2018 Aug; 1-10. doi https://doi.org/10.1007/s40272-018-0307-z
- ↑ 9.0 9.1 https://www.uniprot.org/uniprot/P00451 [11.01.2019]
- ↑ 10.0 10.1 10.2 http://www.rcsb.org/structure/3CDZ [11.01.2019]
- ↑ 11.0 11.1 11.2 11.3 Toole JJ, Pittman DD, Orr EC, Murtha P, Wasley LC & Kaufman RJ. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proceedings of the National Academy of Sciences. 1986 Aug; 83(16): 5939-5942. PMID: 3016730 doi https://doi.org/10.1073/pnas.83.16.5939
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Bihoreau N, Fontaine-Aupart MP, Lehegarat A, Desmadril M, Yon JM. First determination of the secondary structure of purified factor VIII light chain. Biochem J. 1992 Nov; 288 ( Pt 1): 35-40. PMID:1445279 doi: 10.1042/bj2880035
- ↑ Lenting PJ, Pegon JN, Christophe OD, Denis CV. Factor VIII and von Willebrand factor – too sweet for their own good. Haemophilia. 2010 June; 16(Suppl. 5), 194–199. PMID: 20590881 doi: https://doi.org/10.1111/j.1365-2516.2010.02320.x
- ↑ 14.0 14.1 Apweiler R, Hermjakob H,Sharon N.On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database.Biochimica et Biophysica Acta (BBA)-General Subjects.1999 Dec; 1473(1), 4-8.PMID: 10580125 doi: 10.1016/s0304-4165(99)00165-8
- ↑ Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001 Mar, 291(5512), 2364-2369 doi:10.1126/science.291.5512.2364
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/439174 [25.06.2019]
- ↑ https://en.wikipedia.org/wiki/Mannose [25.06.2019]
- ↑ 18.0 18.1 18.2 18.3 18.4 Srivastava A, Brewer AK, Mauser‐Bunschoten EP, Key NS, Kitchen S, Llinas A, Ludlam CA, Mahlangu JN, Mulder K, Poon MC & Street A. Guidelines for the management of hemophilia. Haemophilia. 2013 Jan; 19(1): e1-e47. PMID: 22776238 doi: 10.1111/j.1365-2516.2012.02909.x
- ↑ 19.0 19.1 19.2 Konkle BA, Huston H & Fletcher SH. Hemophilia A, Synonym: Factor VIII Deficiency. Gene Rewiews. 2017 Jun. PMID: 20301578
- ↑ White GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia, Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001 Mar; 85(3): 560. PMID: 11307831