User:Ashley Crotteau/Sandbox1
From Proteopedia
< User:Ashley Crotteau(Difference between revisions)
| (14 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<StructureSection load= '1o9s' size='350' frame='true' side='right' caption='H. sapiens KMT 1o9s' scene='81/811707/Overall_structure/1'> | <StructureSection load= '1o9s' size='350' frame='true' side='right' caption='H. sapiens KMT 1o9s' scene='81/811707/Overall_structure/1'> | ||
| + | |||
==Introduction== | ==Introduction== | ||
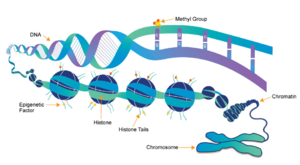
| + | [[Image:Epigentics pic.png|300 px|right|thumb|Figure 1: Epigenetic overview. Showing the methylation of histones and how this affects DNA packing.<ref name="Epigenetics" />]] | ||
| - | [https://en.wikipedia.org/wiki/Histone Histones] are a family of proteins that condense DNA into chromatin, and is an octamer composed of two of each protein core; H2A, H2B, H3, and H4. Histones are a globular protein, that | + | [https://en.wikipedia.org/wiki/Histone Histones] are a family of proteins that condense DNA into chromatin, and is an octamer composed of two of each protein core; H2A, H2B, H3, and H4. Histones are a globular protein, that have N- or C-terminal tails. These tails can be subjected to modifications by enzymes. Methylation of histones is one of the four common histone modifications. Methylation is most common on long tails of H3 and H4 due to the tail being able to enter the active site.<ref name ="DesJarlais" /> A histone can be mono-, di-, or tri- methylated. Once the histone is methylated, the DNA goes from tightly bound heterochromatin to loosely packed euchromatin shown in Figure 1. The euchromatin allows RNA pol II to bind to the DNA and start transcription. <ref name="Marino" /> Histone methylation is a major epigenetic marker, which can be passed down from generation to generation. An [https://www.whatisepigenetics.com/fundamentals/ epigenetic marker] affects the way that genes are expressed, and can activate or repress DNA. Histone methylation is a major epigenetic marker because it has the ability to change heterochromatin to euchromatin, and vice versa. Alterations in markers have been associated with many diseases.<ref name="Xiao" /> Lysine Methyltrasferase SET7/9 (KMT) is an enzyme that methylates the histone 3 lysine 4 (H3K4) and plays a important role in the transcription of DNA in ''Homo sapiens''.<ref name="Biterge" /> The methylation of H3K4 results in transcriptional activation.<ref name="Biterge" /> The specific methylation of H3K4 does not result in a change in charge because it is a nonpolar group being added to the lysine. A change in charge could result in tighter bound heterochromatin. |
==Structure== | ==Structure== | ||
| - | The human lysine methyltransferase(HKMT) SET7/9 is 366 amino acids long that comes from ''H. sapiens''. Structure has two proteins in the asymmetric unit, yet acts as a monomer. HKMT will crystalize as a dimer. The structure is composed of the ΔSET7/9 domain. There is a two-domain architecture containing a conserved anti-parallel 𝛃-barrel and an unusual knot-like structure that creates the active site. It also contains a cofactor, SAM, that plays a role in the active site. HKMT only monomethylates H3K4, because the tyrosine rich active site does not allow di- or tri- methylation. | + | The human lysine methyltransferase(HKMT) SET7/9 is 366 amino acids long that comes from ''H. sapiens''. Structure has two proteins in the asymmetric unit, yet acts as a monomer. HKMT will crystalize as a dimer. The structure is composed of the ΔSET7/9 domain. There is a two-domain architecture containing a conserved anti-parallel 𝛃-barrel and an unusual knot-like structure that creates the active site. It also contains a cofactor, SAM, that plays a role in the active site.<ref name="Biterge" /> HKMT only monomethylates H3K4, because the tyrosine rich active site does not allow for di- or tri- methylation. |
===SET Domain=== | ===SET Domain=== | ||
| - | The ΔSET7/9 consists of the SET domain along with the pre- and post-SET regions.<ref name="Schubert" /><ref name="Yeates" /> The pre- and post-SET regions are adjacent to SET domain and are cysteine rich.<ref name="Schubert" /><ref name="Yeates" /> The pre-SET cysteine region is located near the N-terminal where the post-SET region is located near the C-terminal of the domain.<ref name="Schubert" /><ref name="Yeates" /> These regions are said to play an important role in substrate recognition and enzymatic activity.<ref name="Schubert" /><ref name="Yeates" /> The cysteine regions are not shown in the crystal structure as only residues 117-366 were crystalized.<ref name="Xiao" /> | + | The ΔSET7/9 consists of the [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1273623/ SET domain] along with the pre- and post-SET regions.<ref name="Schubert" /><ref name="Yeates" /> The pre- and post-SET regions are adjacent to SET domain and are cysteine rich.<ref name="Schubert" /><ref name="Yeates" /> The pre-SET cysteine region is located near the N-terminal where the post-SET region is located near the C-terminal of the domain.<ref name="Schubert" /><ref name="Yeates" /> These regions are said to play an important role in substrate recognition and enzymatic activity.<ref name="Schubert" /><ref name="Yeates" /> The cysteine regions are not shown in the crystal structure as only residues 117-366 were crystalized.<ref name="Xiao" /> |
| - | The SET domain is mostly defined by | + | The SET domain is mostly defined by turns and loops, which connect secondary structures, with the few <scene name='81/811707/Beta_sheets/4'>antiparallel β-sheets</scene>.<ref name="Schubert" /> <scene name='81/811707/Beta-hairpin/3'>Residues 337-349</scene> form a β-hairpin that sticks out at a right angle to the surface of the enzyme.<ref name="Xiao" /> This hairpin stabilizes the conformation of tyrosine residues.<ref name="Xiao" /> The following three residues (<scene name='81/811707/Sharp_bend/4'>350-352</scene>) accommodate a unique sharp bend in the peptide chain and the end of the protein takes on an <scene name='81/811707/C-term_alpha_helix/2'>α-helical conformation</scene>.<ref name="Xiao" /> This alpha helix packs against a beta sheet and orients the cofactor towards the active site.<ref name="Xiao" /> The two most defining features of the SET domain are the C-terminal tyrosine and the <scene name='81/811707/Variable_knot/3'>knot-like fold</scene>. These two components have been recognized to be essential for S-adenosyl-L-methionine(SAM) binding and catalysis, which is shown as <scene name='81/811707/Sam_isolated/3'>S-adenosyl-L-homocysteine</scene>(SAH) in the structure after methylation of the histone.<ref name="Schubert" /> <ref name="Yeates" /> <ref name="Huang" /> The knot-like fold contains the binding sites for the cofactor SAM and the peptide substrate.<ref name="Licciardello" /> |
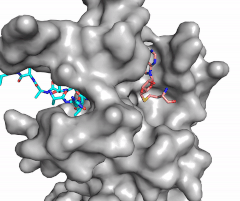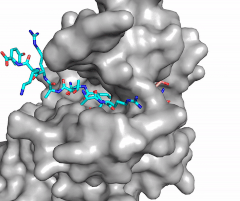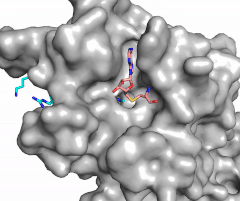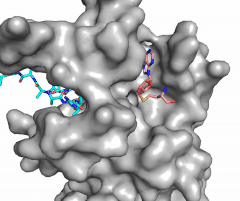
| - | [[Image:Methyl1.gif|300 px|right|thumb|Figure | + | [[Image:Methyl1.gif|300 px|right|thumb|Figure 2: Image of substrate bound on one side of SET7/9 (PDB 1o9s) with the lysine target in the active site channel (cyan) and S-adenosyl homocysteine (pink) bound on the opposite face of the enzyme]] |
===Active Site and Channel=== | ===Active Site and Channel=== | ||
| - | The most notable feature of the HKMT is the presence of the lysine access channel as the active site. The cofactor and <scene name='81/811707/ | + | The most notable feature of the HKMT is the presence of the lysine access channel as the active site. The cofactor and <scene name='81/811707/Peptide/1'>peptide</scene> substrate are located on opposite sides of the SET domain but are connected through this narrow channel (Figure 2).<ref name="Xiao" /> This channel allows these two components to interact and complete the methyltransfer. The active site in general is considerably tyrosine rich. Residues Tyr245, His297, Ser268, Tyr305, Tyr335, and Tyr337 all help to shape the <scene name='81/811707/Stick_active_site/3'>active site</scene> and the channel.<ref name="Xiao" /> The cofactor involved, SAM, provides the methyl for methylation of the lysine on its sulfur atom. |
| - | The beta hairpin stabilizes the <scene name='81/811707/Beta_hairpin_stabilizing_tyrs/2'>conformation of Tyr335 and Tyr337</scene>, while also shaping one side of the channel which the peptide binds to.<ref name="Xiao" /> The | + | The beta hairpin stabilizes the <scene name='81/811707/Beta_hairpin_stabilizing_tyrs/2'>conformation of Tyr335 and Tyr337</scene>, while also shaping one side of the channel which the peptide binds to.<ref name="Xiao" /> The peptide binding site is composed of residues 255-268.<ref name="Xiao" /> Lysine would have trouble coming down into the active site in its charged form, but it is enabled by the faces of the flanking tyrosines.<ref name="Xiao" /> The lysine is oriented so that the amine-methyl bond is pointed towards the sulfur on SAM so that it can provide the methyl. There is an important <scene name='81/811707/Water_interaction/1'>water</scene> in the active site that accepts a hydrogen bond from the lysine substrate, shifting the lone pair on the nitrogen towards the sulfur of SAM. <ref name="Xiao" /> |
== Function == | == Function == | ||
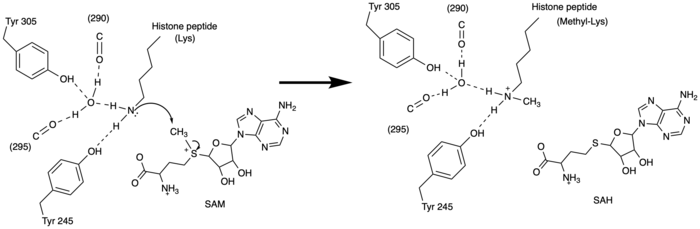
| - | [[Image:KMT mechanism mm.png|700 px|right|thumb|Figure | + | [[Image:KMT mechanism mm.png|700 px|right|thumb|Figure 3: Histone Methylation by HKMT Mechanism]] |
| - | The N of the amine from the lysine serves as a nucleophile that attacks the electrophilic CH<sub>3</sub> that is present in the AdoMet (SAM). The sulfur that the CH<sub>3</sub> is attached to pulls the electrons towards itself to weaken the bond between the sulfur and the carbon. This weak bond allows for the N to break that bond and take the methyl group. The N on the lysine is being stabilized by Tyr residues and a water molecule (Figure | + | The N of the amine from the lysine serves as a nucleophile that attacks the electrophilic CH<sub>3</sub> that is present in the AdoMet (SAM). The sulfur that the CH<sub>3</sub> is attached to pulls the electrons towards itself to weaken the bond between the sulfur and the carbon. This weak bond allows for the N to break that bond and take the methyl group. The N on the lysine is being stabilized by Tyr residues and a water molecule (Figure 3). This allows the N to accept the methyl and take up that positive charge. |
| - | The tyrosine conserved regions of KMT have been found to be vital for only monomethylation. Multiple studies have found that a mutation to any of the conserved tyrosine residues does still monomethylate SAM, but it also creates di-methylation and tri- methylation of SAM.<ref name="Del Rizzo" /> In a recent study, Y245A and Y305F were created through site-directed mutagenesis.<ref name="Del Rizzo" /> Due to size, Ala245 was found to create a larger opening of the channel than tyrosine, which allowed for further methylation of SAM. <ref name="Del Rizzo" /> However, Y305F also showed the same characteristics of di- and tri-methylation, most likely due to a decrease of tyrosine residues shaping the narrow channel.<ref name="Del Rizzo" /> As there are four invariant tyrosine residues (Tyr305, Tyr245, Tyr335, Tyr337) in the active site, this finding indicates that the function of KMT is dependent on the presence of tyrosine residues in the active site. <ref name="Del Rizzo" /> Although only two of the tyrosines are interacting in the mechanism (Figure | + | The tyrosine conserved regions of KMT have been found to be vital for only monomethylation. Multiple studies have found that a mutation to any of the conserved tyrosine residues does still monomethylate SAM, but it also creates di-methylation and tri- methylation of SAM.<ref name="Del Rizzo" /> In a recent study, Y245A and Y305F were created through site-directed mutagenesis.<ref name="Del Rizzo" /> Due to size, Ala245 was found to create a larger opening of the channel than tyrosine, which allowed for further methylation of SAM. <ref name="Del Rizzo" /> However, Y305F also showed the same characteristics of di- and tri-methylation, most likely due to a decrease of tyrosine residues shaping the narrow channel.<ref name="Del Rizzo" /> As there are four invariant tyrosine residues (Tyr305, Tyr245, Tyr335, Tyr337) in the active site, this finding indicates that the function of KMT is dependent on the presence of tyrosine residues in the active site. <ref name="Del Rizzo" /> Although only two of the tyrosines are interacting in the mechanism (Figure 3), the other two are shaping the narrow channel. Without these extra tyrosines, Tyr335 and Tyr337, the channel is not as narrow and can therefore di- and tri- methylate lysine. |
== Relevance == | == Relevance == | ||
===Renal Fibrosis=== | ===Renal Fibrosis=== | ||
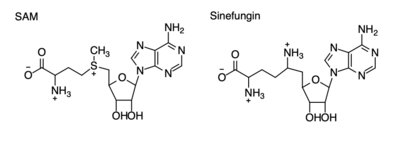
| - | [[Image:Sam_vs_sinefungin.png|400 px|right|thumb|Figure | + | [[Image:Sam_vs_sinefungin.png|400 px|right|thumb|Figure 4: Structural differences between SAM and Sinefungin]] |
| - | The inhibition of KMT SET 7/9 has been found to enhance renal fibrosis.<ref name="Sun" /> H3K4 methylation activates the transcription of fibrotic genes, and the suppression of the H3K4 methylation was found to enhance renal fibrosis in a mouse model.<ref name="Sun" /> Sinefungin, a competitive methyltransferase inhibitor, binds to KMT to inhibit SAM.<ref name="Sun" /> SAM and Sinefungin have similar structures (Figure | + | The inhibition of KMT SET 7/9 has been found to enhance renal fibrosis.<ref name="Sun" /> H3K4 methylation activates the transcription of fibrotic genes, and the suppression of the H3K4 methylation was found to enhance renal fibrosis in a mouse model.<ref name="Sun" /> Sinefungin, a competitive methyltransferase inhibitor, binds to KMT to inhibit SAM.<ref name="Sun" /> SAM and Sinefungin have similar structures (Figure 4), differing only with the removal of a sulfide and the replacement of a methyl to an amine. The amine replaces the methyl donor for the reaction of KMT, inhibiting the reaction and preventing methylation. Without the methylation of H3K4, the transcription of fibrotic genes is deactivated, leading to renal fibrosis.<ref name="Sun" /> |
===Cancer=== | ===Cancer=== | ||
| - | Many studies have shown that lysine methyltransferases lead to the inhibition of cancers, therefore they are being studied as possible cancer therapy treatments. Lysine methylation contributes to the inactivation of a tumor suppressor gene. Due to this, KMTs are being studied as possible biomarkers for the detection of cancers. Inhibitors of KMT, such as Sinefungin (Figure | + | Many studies have shown that lysine methyltransferases lead to the inhibition of cancers, therefore they are being studied as possible cancer therapy treatments. Lysine methylation contributes to the inactivation of a tumor suppressor gene. Due to this, KMTs are being studied as possible biomarkers for the detection of cancers. Inhibitors of KMT, such as Sinefungin (Figure 4), were used in a study to observe KMT’s effect on cancerous cells. It was found that when KMT is deregulated, tumor behavior increases due to a lack of methylation, indicating the importance of the histone methylation. <ref name=Xuejiao /> |
</StructureSection> | </StructureSection> | ||
| Line 59: | Line 61: | ||
<ref name="Marino">PMID: 16209651 </ref> | <ref name="Marino">PMID: 16209651 </ref> | ||
<ref name="Biterge" <ref> DOI: 10.15406/mojcsr.2016.03.00047</ref> | <ref name="Biterge" <ref> DOI: 10.15406/mojcsr.2016.03.00047</ref> | ||
| + | <ref name="Epigenetics" <ref>https://www.whatisepigenetics.com/fundamentals/</ref> | ||
<references/> | <references/> | ||
Current revision
H. sapiens Lysine Methyltransferase, SET 7/9
| |||||||||||
References
[4] [9] [6] [7] [8] [10] [11] [12] [2] [3] [5] [1]
- ↑ 1.0 1.1 https://www.whatisepigenetics.com/fundamentals/
- ↑ 2.0 2.1 DesJarlais R, Tummino PJ. Role of Histone-Modifying Enzymes and Their Complexes in Regulation of Chromatin Biology. Biochemistry. 2016 Mar 22;55(11):1584-99. doi: 10.1021/acs.biochem.5b01210. Epub , 2016 Jan 26. PMID:26745824 doi:http://dx.doi.org/10.1021/acs.biochem.5b01210
- ↑ 3.0 3.1 Marino-Ramirez L, Kann MG, Shoemaker BA, Landsman D. Histone structure and nucleosome stability. Expert Rev Proteomics. 2005 Oct;2(5):719-29. PMID:16209651 doi:http://dx.doi.org/10.1586/14789450.2.5.719
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ 5.0 5.1 5.2 5.3 doi: https://dx.doi.org/10.15406/mojcsr.2016.03.00047
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci. 2003 Jun;28(6):329-35. PMID:12826405
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Yeates TO. Structures of SET domain proteins: protein lysine methyltransferases make their mark. Cell. 2002 Oct 4;111(1):5-7. PMID:12372294
- ↑ 8.0 8.1 Huang S, Shao G, Liu L. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J Biol Chem. 1998 Jun 26;273(26):15933-9. PMID:9632640
- ↑ 9.0 9.1 doi: https://dx.doi.org/10.1016/C2014-0-02189-2
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Del Rizzo PA, Couture JF, Dirk LM, Strunk BS, Roiko MS, Brunzelle JS, Houtz RL, Trievel RC. SET7/9 catalytic mutants reveal the role of active site water molecules in lysine multiple methylation. J Biol Chem. 2010 Oct 8;285(41):31849-58. Epub 2010 Aug 1. PMID:20675860 doi:http://dx.doi.org/10.1074/jbc.M110.114587
- ↑ 11.0 11.1 11.2 11.3 11.4 Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010 Dec;21(12):2069-80. doi: 10.1681/ASN.2010060633. Epub 2010, Oct 7. PMID:20930066 doi:http://dx.doi.org/10.1681/ASN.2010060633
- ↑ 12.0 12.1 Tian X, Zhang S, Liu HM, Zhang YB, Blair CA, Mercola D, Sassone-Corsi P, Zi X. Histone lysine-specific methyltransferases and demethylases in carcinogenesis: new targets for cancer therapy and prevention. Curr Cancer Drug Targets. 2013 Jun;13(5):558-79. doi:, 10.2174/1568009611313050007. PMID:23713993 doi:http://dx.doi.org/10.2174/1568009611313050007
Student Contributors
Ashley Crotteau
Parker Hiday
Lauren Allman