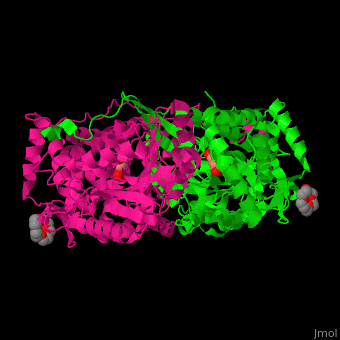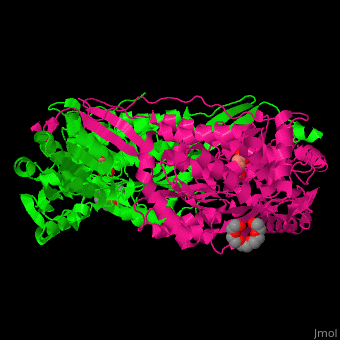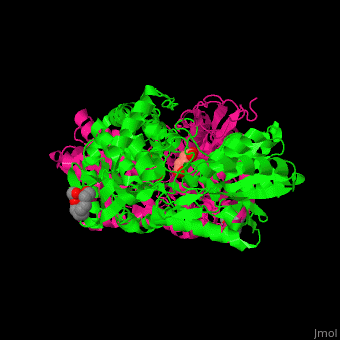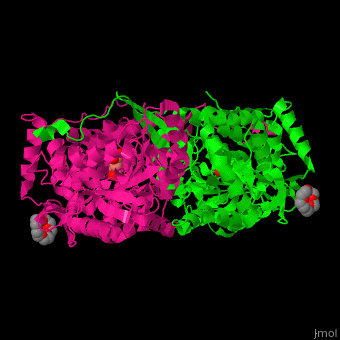
Pyrroline-5-carboxylate dehydrogenase
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Mouse pyrroline-5-carboxylate dehydrogenase dimer complex with glutarate and PEG400 (PDB entry [[4lh3]])' scene='51/510199/Cv1/1'> | <StructureSection load='' size='350' side='right' caption='Mouse pyrroline-5-carboxylate dehydrogenase dimer complex with glutarate and PEG400 (PDB entry [[4lh3]])' scene='51/510199/Cv1/1'> | ||
== Function == | == Function == | ||
| - | '''Pyrroline-5-carboxylate dehydrogenase''' (PCD) catalyzes the reversible dehydrogenation of 1-pyrroline-5-carboxylate to glutamate using NAD or NADP as cofactors. PCD participates in glutamate, proline and arginine metabolism. PCD is the second enzyme in proline degradation hence it is important in stress conditions when plants accumulate proline<ref>PMID:15548746</ref>. | + | '''Pyrroline-5-carboxylate dehydrogenase''' (PCD) catalyzes the reversible dehydrogenation of 1-pyrroline-5-carboxylate to glutamate using NAD or NADP as cofactors. PCD participates in glutamate, proline and arginine metabolism. PCD is the second enzyme in proline degradation hence it is important in stress conditions when plants accumulate proline<ref>PMID:15548746</ref>. See also [[Aldehyde dehydrogenase]]. |
== Disease == | == Disease == | ||
| Line 18: | Line 18: | ||
*Pyrroline-5-carboxylate dehydrogenase | *Pyrroline-5-carboxylate dehydrogenase | ||
| + | **[[3v9g]], [[4oe5]] – hPCD – human<BR /> | ||
| + | **[[3v9h]], [[3v9i]] – hPCD (mutant) <BR /> | ||
| + | **[[3v9j]], [[4lgz]], [[4e3x]] – mPCD – mouse<BR /> | ||
| + | **[[4oe6]] – yPCD – yeast<br /> | ||
**[[1uzb]] - TtPCD –''Termus thermophilus''<BR /> | **[[1uzb]] - TtPCD –''Termus thermophilus''<BR /> | ||
**[[4k57]] - TtPCD (mutant)<BR /> | **[[4k57]] - TtPCD (mutant)<BR /> | ||
| - | **[[4oe6]] – yPCD – yeast<br /> | ||
**[[3qan]] – PCD – ''Bacillus halodurans''<BR /> | **[[3qan]] – PCD – ''Bacillus halodurans''<BR /> | ||
**[[3rjl]] - PCD – ''Bacillus licheniformis''<BR /> | **[[3rjl]] - PCD – ''Bacillus licheniformis''<BR /> | ||
| - | **[[3v9g]], [[4oe5]] – hPCD – human<BR /> | ||
| - | **[[3v9h]], [[3v9i]] – hPCD (mutant) <BR /> | ||
| - | **[[3v9j]], [[4lgz]], [[4e3x]] – mPCD – mouse<BR /> | ||
**[[4idm]] – MtPCD – ''Mycobacterium tuberculosis''<br /> | **[[4idm]] – MtPCD – ''Mycobacterium tuberculosis''<br /> | ||
**[[4ids]], [[4jdc]] - MtPCD (mutant)<br /> | **[[4ids]], [[4jdc]] - MtPCD (mutant)<br /> | ||
| Line 31: | Line 31: | ||
*PCD complex with cofactor | *PCD complex with cofactor | ||
| - | **[[ | + | **[[3v9l]] - mPCD + NAD<BR /> |
| - | **[[ | + | **[[7mer]], [[7mes]] – m1-PCD + NAD + hydroxyl-proline<br /> |
**[[4oe4]] - yPCD + NAD <BR /> | **[[4oe4]] - yPCD + NAD <BR /> | ||
| + | **[[2bhp]], [[2bja]] – TtPCD + NAD<BR /> | ||
**[[2ehq]] - TtPCD + NADP<BR /> | **[[2ehq]] - TtPCD + NADP<BR /> | ||
| - | **[[3v9l]] - mPCD + NAD<BR /> | ||
**[[2bhq]] - TtPCD + glutamate + NAD<BR /> | **[[2bhq]] - TtPCD + glutamate + NAD<BR /> | ||
**[[2bjk]] - TtPCD + citrate + NAD<BR /> | **[[2bjk]] - TtPCD + citrate + NAD<BR /> | ||
| Line 43: | Line 43: | ||
**[[2eiw]], [[2ej6]], [[2j40]] - TtPCD + proline + NAD<BR /> | **[[2eiw]], [[2ej6]], [[2j40]] - TtPCD + proline + NAD<BR /> | ||
**[[2j5n]] - TtPCD + glycine + NAD<BR /> | **[[2j5n]] - TtPCD + glycine + NAD<BR /> | ||
| + | **[[4ihi]] - MtPCD + NAD<BR /> | ||
**[[4ns3]] - MtPCD (mutant) + cobalamin + NAD<br /> | **[[4ns3]] - MtPCD (mutant) + cobalamin + NAD<br /> | ||
| Line 51: | Line 52: | ||
**[[4lh2]] - mPCD + succinate<br /> | **[[4lh2]] - mPCD + succinate<br /> | ||
**[[4lh3]] - mPCD + glutarate<br /> | **[[4lh3]] - mPCD + glutarate<br /> | ||
| - | **[[3v9k | + | **[[3v9k]] - mPCD + proline |
**[[4lem]] - MtPCD (mutant) + cobalamin<br /> | **[[4lem]] - MtPCD (mutant) + cobalamin<br /> | ||
**[[2iy6]] - TtPCD + citrate<br /> | **[[2iy6]] - TtPCD + citrate<br /> | ||
Current revision
| |||||||||||
3D structures of pyrroline-5-carboxylate dehydrogenase
Updated on 09-February-2022
References
- ↑ Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommer WB. The role of [Delta]1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell. 2004 Dec;16(12):3413-25. Epub 2004 Nov 17. PMID:15548746 doi:http://dx.doi.org/10.1105/tpc.104.023622
- ↑ Geraghty MT, Vaughn D, Nicholson AJ, Lin WW, Jimenez-Sanchez G, Obie C, Flynn MP, Valle D, Hu CA. Mutations in the Delta1-pyrroline 5-carboxylate dehydrogenase gene cause type II hyperprolinemia. Hum Mol Genet. 1998 Sep;7(9):1411-5. PMID:9700195
- ↑ Pemberton TA, Tanner JJ. Structural basis of substrate selectivity of Delta-pyrroline-5-carboxylate dehydrogenase (ALDH4A1): Semialdehyde chain length. Arch Biochem Biophys. 2013 Aug 6. pii: S0003-9861(13)00231-2. doi:, 10.1016/j.abb.2013.07.024. PMID:23928095 doi:10.1016/j.abb.2013.07.024